By going through these Maharashtra State Board 12th Science Chemistry Notes Chapter 3 Ionic Equilibria students can recall all the concepts quickly.
Maharashtra State Board 12th Chemistry Notes Chapter 3 Ionic Equilibria
→ Formulae on Ostwald’s dilution law:
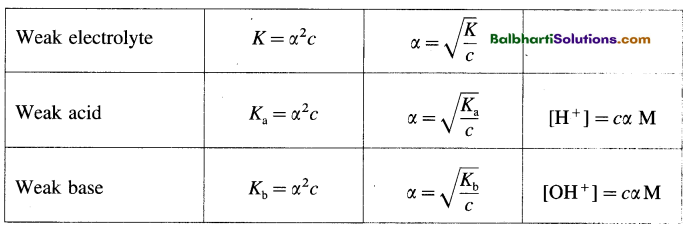
→ Kw = [H3O+] × [OH–], at 25°C Kw = 1 × 10-14
→ pH = -log10[H+]; pOH = -log10[OH–]
![]()
→ pH + pOH = 14
→ Acidic buffer solution: pH = PKa + log10 \(\frac { [salt] }{ [acid] }\)
→ Basic buffer solution: pOH = pKb + log10 \(\frac { [salt] }{ [acid] }\)
→ PKa = – log10 Ka; pKb = – log10 Kb
→ Solubility product:
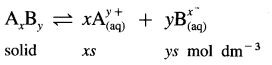
ksp = (xs)x (ys)y = xxyy x (S)x+y