By going through these Maharashtra State Board 12th Science Chemistry Notes Chapter 16 Green Chemistry and Nanochemistry students can recall all the concepts quickly.
Maharashtra State Board 12th Chemistry Notes Chapter 16 Green Chemistry and Nanochemistry
Green chemistry: Green chemistry is the use of chemistry for pollution prevention and it designs
the chemical products and processes that reduce or eliminate the use of generation of hazardous
substances.
Sustainable development: Sustainable development is the development that meets the needs of
the present, without compromising the ability of future generations to meet their own needs.
Twelve principles of green chemistry: Twelve principles of green chemistry were proposed by Paul T. Anastas and John Warner.
![]()
12 Principles of Green Chemistry –
- Prevention of waste or by-products
- Atom economy
- Less hazardous chemical synthesis
- Designing safer chemicals
- Use safer solvent and auxiliaries
- Design for energy efficiency
- Use of renewable feedstocks
- Reduce derivatives (Minimization of steps)
- Use of catalysis
- Design for degradation
- Real-time analysis for pollution prevention
- Safer chemistry for accident prevention
The role of Green chemistry:
- Promoting innovative chemical technology to design, manufacture, and use chemical products that eliminate the generation of hazardous chemicals.
- The capital expenditure required for the prevention of pollution is controlled by the use of green chemistry.
- Green chemistry helps to protect the presence of ozone in the stratosphere.
- Global warming (The greenhouse effect) is controlled by green chemistry.
Introduction to nanochemistry:
(i) Nanoscience: Nanoscience is the study of phenomena and manipulation of materials at atomic, molecular, and macromolecular scales where properties differ significantly from those at a larger scale.
(ii) Nanotechnology: Nanotechnology is the design, characterization, production, and application of structures, devices, and systems by controlling shape and size at the nanometer scale (1 -100 nm) (1 nm = 10-9 m). Today from clothes to computer hard drives to DVD, CD players, and even cleaning products, nanotechnology plays a big part in the manufacturing of materials.
(iii) Nanomaterial: The nanomaterial is a material having structural components with at least one dimension in the nanometer scale that is 1-100 nm. Nanomaterials are larger than single atoms but smaller than bacteria and cells. These may be nanoparticles, nanowires, nanotubes, and thin films according to dimensions. They can be further classified as zero, one, and two nanomaterial dimensions.
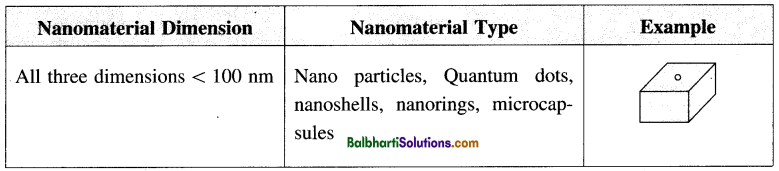
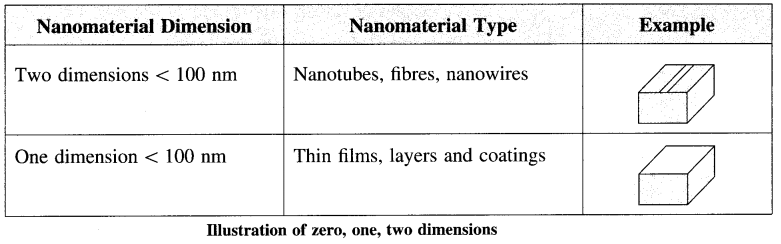
(iv) Nanochemistry: It is the combination of chemistry and nanoscience. It deals with designing and synthesis of materials of nanoscale with different sizes and shape, structure and composition, and their organization into functional architectures. Nanochemistry is used in chemical, physical, material science, as well as engineering, biological and medical applications.
Characteristic features of nanoparticles: Characteristic features of nanoparticles are colour, surface area, catalytic activity, thermal properties, mechanical properties, and electrical conductivity.
Synthesis of nanomaterials: (1) Bottom-up approach (2) Top-down approach.
Wet chemical synthesis of nanomaterials: A sol-gel process is an inorganic polymerization reaction. It is generally carried out at room temperature and includes four steps: hydrolysis, polycondensation, drying, and thermal decomposition. This method is widely used to prepare oxidic materials.
The reactions involved in the sol-gel process are as follows :
MOR + H2O → MOH + ROH (hydrolysis)
metal alkoxide
MOH + ROM → M-O-M + ROH (condensation)
![]()
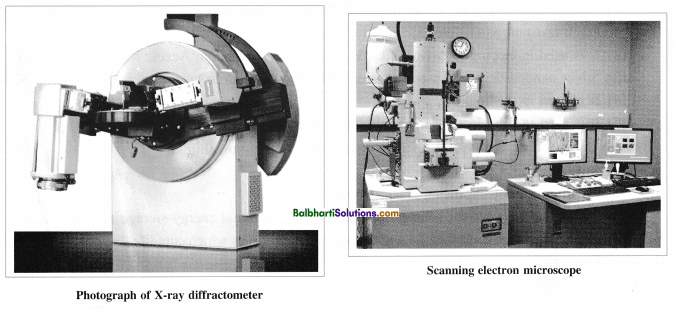
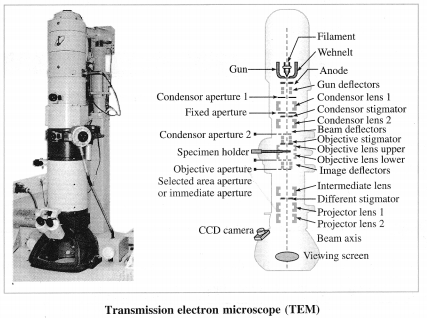
Analysis or characterization of nanomaterials: The nanomaterial synthesized is analyzed using the following techniques.
| Name of Technique | Instrument used | Information |
| 1. UV-visible spectroscopy | UV-visible spectrophotometer | Preliminary confirmation of formation of nanoparticles |
| 2. X-ray Diffraction (XRD) | X-ray diffractometer | Particle size, crystal structure, geometry |
| 3. Scanning electron microscopy | Scanning electron microscope (SEM) | Structure of surface of the material that is morphology |
| 4. Transmission electron microscopy | Transmission electron microscope (TEM) | Particle size |
| 5. FTIR Fourier transform infrared spectroscopy | Fourier transform infrared spectrophotometer | Absorption of functional groups, Binding nature. |
Different types of nanomaterials which can be synthesized are shown in the following figures:

Applications of nanoparticles:
- Nanoparticles are used in the manufacture of scratchproof eyeglasses, transport, sunscreen, crack-resistant paints, etc.
- It is used in electronic devices like Magnetoresistive Random Access Memory (MRAM).
- Silver nanoparticles are used in water purification systems to get safe drinking water.
- It is used in medicines.
- It is used in self-cleaning materials (lotus effect).
![]()
Advantages and disadvantages of nanoparticles and nanotechnology :
Advantages:
- Revolution in electronics and computing.
- Nanotechnology makes solar power more economical and energy storage more efficient.
- Nanotechnology is used in the manufacture of smart drugs which cure life-threatening diseases faster and without side effects.
Disadvantages :
- Nanotechnology causes environmental pollution which is dangerous for living organisms.
- Nanoparticles can cause lung damage.