By going through these Maharashtra State Board 12th Science Chemistry Notes Chapter 2 Solutions students can recall all the concepts quickly.
Maharashtra State Board 12th Chemistry Notes Chapter 2 Solutions
→ Henry’s law : S = KHP
→ Raoult’s law : Psoln = x1P0
→ Mole fractions, x1 + x2 = 1
→ Psoln = P°1x1 + P°2x2
→ Psoln = (P°2 – P°1)x2 + P°1
![]()
→ Mole fractions of components in vapour phase : y1 = \(\frac{x_{1} P_{1}^{0}}{P}\) and y2 = \(\frac{x_{2} P_{2}^{0}}{P}\)
→ For ideal solutions : ΔVmix = 0; ΔmixH = 0
→ Relative lowering of vapour pressure = \(\frac{P^{0}-P}{P^{0}}\), x2 = \(\frac{P^{0}-P}{P^{0}}\)
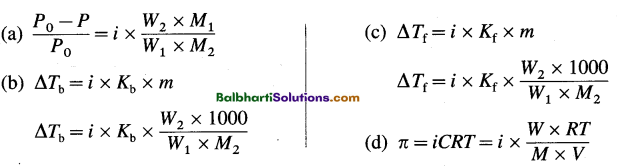
→ \(\frac{P^{0}-P}{P^{0}}=\frac{W_{2} \times M_{1}}{W_{1} \times M_{2}}\)
→ Δ Tb = Kb x m and Δ Tf — Kf x m
→ ΔTb = Kb x \(\frac{W_{2} \times 1000}{W_{1} \times M_{2}}\)
→ ΔTf = Kf x \(\frac{W_{2} \times 1000}{W_{1} \times M_{2}}\)
→ π = cRT and π = \(\frac{W R T}{M \times V}\)
→ i = \(\frac{Colligative property of electrolyte solution}{Colligative property of nonelectrolyte solution of the same concentration}\)
→ Van’t Hoff factor (i) = \(\frac{\Delta T_{\mathrm{b}(\mathrm{ob})}}{\Delta T_{\mathrm{b}(\mathrm{th})}}=\frac{\Delta P_{(\mathrm{ob})}}{\Delta P_{(\mathrm{th})}}=\frac{\Delta T_{\mathrm{f}(\mathrm{ob})}}{\Delta T_{\mathrm{f}(\mathrm{th})}}=\frac{\pi_{\mathrm{ob}}}{\pi_{\mathrm{th}}}=\frac{M_{\mathrm{th}}}{M_{\mathrm{ob}}}\)
→ Colligative properties considering van’t Hoff factor :
![]()
→ α = \(\frac{i-1}{n-1}\) (for dissociation)