Aldehydes, Ketones and Carboxylic Acids Class 12 Exercise Question Answers Solutions Maharashtra Board
Balbharti Maharashtra State Board 12th Chemistry Textbook Solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids Textbook Exercise Questions and Answers.
Class 12 Chemistry Chapter 12 Exercise Solutions Maharashtra Board
Chemistry Class 12 Chapter 12 Exercise Solutions
1. Choose the most correct option.
Question i.
In the following resonating structures A and B, the number of unshared electrons in valence shell present on oxygen respectively are
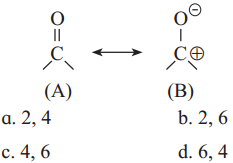
Answer:
c. 4, 6
![]()
Question ii.
In the Wolf -Kishner reduction, alkyl aryl ketones are reduced to alkylbenzenes. During this change, ketones are first converted into
a. acids
b. alcohols
c. hydrazones
d. alkenes
Answer:
c. hydrazones
Question iii.
Aldol condensation is
a. electrophilic substitution reaction
b. nucleophilic substitution reaction
c. elimination reaction
d. addition – elimination reaction
Answer:
d. addition-elimination reaction
Question iv.
Which one of the following has the lowest acidity?
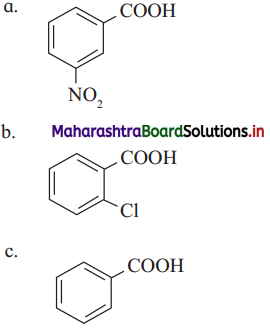
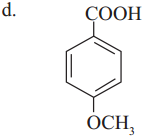
Answer:
(c)
Question v.
Diborane reduces
a. ester group
b. nitro group
c. halo group
d. acid group
Answer:
d. acid group
Question vi.
Benzaldehyde does NOT show positive test with
a. Schiff reagent
b. Tollens’ ragent
c. Sodium bisulphite solution
d. Fehling solution
Answer:
d. Fehling solution
2. Answer the following in one sentence
Question i.
What are aromatic ketones?
Answer:
The compounds in which ![]() group is attached to either two aryl groups or one aryl and one alkyl group are called aromatic ketones.
group is attached to either two aryl groups or one aryl and one alkyl group are called aromatic ketones.
For example :
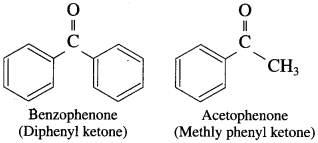
![]()
Question ii.
Is phenylacetic acid an aromatic carboxylic acid?
Answer:
Phenylacetic acid is not an aromatic carboxylic acid.
Question iii.
Write reaction showing conversion of ethanenitrile into ethanol.
Answer:
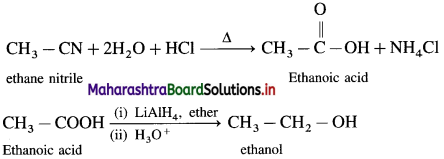
Question iv.
Predict the product of the following reaction:
\(\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{COOCH}_{3} \frac{\mathrm{i} \cdot \mathrm{AlH}(\mathrm{i}-\mathrm{Bu})_{2}}{\text { ii. } \mathrm{H}_{3} \mathrm{O}^{\oplus}}{\longrightarrow} ?\)
Answer:

Question v.
Name the product obtained by reacting toluene with carbon monoxide and hydrogen chloride in presence of anhydrous aluminium chloride.
Answer:
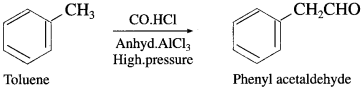
Question vi.
Write reaction showing conversion of Benzonitrile into benzoic acid.
Answer:
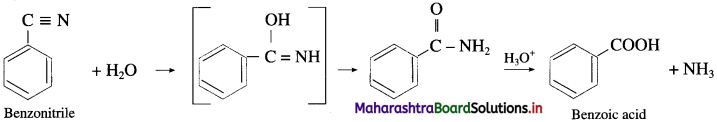
Question vii.
Name the product obtained by the oxidation of 1,2,3,4-tetrahydronaphthalene with acidified potassium permanganate.
Answer:
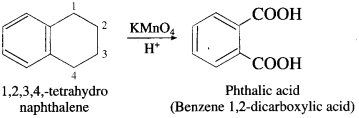
Question viii.
What is formalin?
Answer:
The aqueous solution of formaldehyde (40%) is known as formalin.
Question ix.
Arrange the following compounds in the increasing order of their boiling points : Formaldehyde, ethane, methyl alcohol.
Answer:
Ethane, formaldehyde, methyl alcohol.
![]()
Question x.
Acetic acid is prepared from methyl magnesium bromide and dry ice in presence of dry ether. Name the compound which serves not only reagent but also as cooling agent in the reaction.
Answer:
The cooling agent used in the above reaction is dry ice (O = C = O).
3. Answer in brief.
Question i.
Observe the following equation of reaction of Tollens’ reagent with aldehyde. How do we know that a redox reaction has taken place. Explain.
\(\begin{array}{r}
\mathrm{R}-\mathrm{CHO}+2 \mathrm{Ag}\left(\mathrm{NH}_{3}\right)_{2}^{+}+\mathrm{OH}^{-} \stackrel{\Delta}{\longrightarrow} \\
\mathrm{R}-\mathrm{COO}^{-}+2 \mathrm{Ag} \downarrow+4 \mathrm{NH}_{3}+2 \mathrm{H}_{2} \mathrm{O}
\end{array}\)
Answer:
Tollen’s reagent oxidises acetaldehyde to acetic acid (carboxylate ion) and Ag in Tollen’s reagent complex are reduced to silver. In this reaction, oxidation and reduction takes place simultaneously hence, it is a redox reaction.
Question ii.
Formic acid is stronger than acetic acid. Explain.
Answer:

In acetic acid, the methyl group is an electron-donating group. The acetate ion formed gets destabilized due to the electron releasing effect of methyl group ( +1 effect) which is higher than that of H-atom in the corresponding formic acid. As a result, acetic acid dissociates to a lesser extent. Thus decreasing the acidity of acetic acid.
![]()
Formic acid having lower pKa value than acetic acid. Hence, formic acid is a stronger acid than acetic acid.
Question iii.
What is the action of hydrazine on cyclopentanone in presence of —. KOH in ethylene glycol?
Answer:
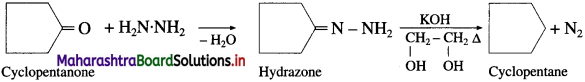
Question iv.
Write reaction showing conversion of Acetaldehyde into acetaldehyde dimethyl acetal.
Answer:

Question v.
Aldehydes are more reactive toward nucleophilic addition reactions than ketones. Explain.
Answer:
The reactivity of aldehydes and keones is due to the polarity of carbonyl group which results in electrophilicity of carbon. The reactivity is further explained on the basis of electronic effect and steric effects.
(1) Influence of electronic effects: A ketone has two electron-donating aJ.kyl groups ( + I effect) bonded to carbonyl carbon which are responsible for decreasing its positive polarity and electrophilicity. In contrast. aldehydes have only electron-donating group bonded to carbonyl carbon. This shows aldehydes are more electrophilic than ketones.
(2) Steric effects : Two bulky alkyl groups in ketone come in the way of the incoming nucleophile. This is called steric hindrance to nucleophilic attack.
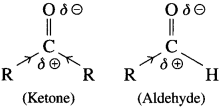
On the other hand. nucleophile can easily attack the carbonyl carbon in aldehyde because has one alkyl group and is less crowded or sterically less hindered.
Hence aldehydes are more reactive and can easily be attacked by nucleophiles.
Question vi.
Write reaction showing the action of the following reagent on propane nitrile
a. Dilute NaOH
b. Dilute HCl ?
Answer:
(1) Action of dil NaOH:
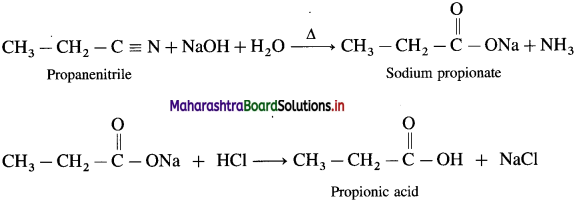
(2) Action of dil HCl:

![]()
Question vi.
Arrange the following carboxylic acids with increasing order of their acidic strength and justify your answer.
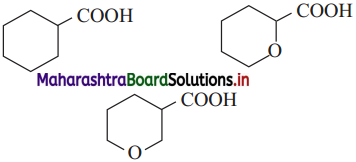
Answer:
The increasing order of acidity will be 1 <3 <2. Acidity depends on mainly two factors : (1) ease of proton release (2) stability of conjugate base formed. In example (3) the ether O exerts a I effect and is closer to COOH group than in 2 (1 effect diminishes). Also the conjugate base formed will be stabilized by the same – I effect by delocalization of charge.
4. Answer the following
Question i.
Write a note on
a. Cannizaro reaction
b. Stephen reaction.
Answer:
(1) The carbon atom adjacent to carbonyl carbon atom is called a-carbon atom (α – C) and the hydrogen atom attached to a-carbon atom is called α-hydrogen atom (α – H).
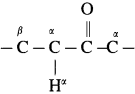
(2) The α-hydrogen of aldehydes and ketones is acidic in nature due to (i) the strong-I effect of carbonyl group (ii) resonance stabilization of the carbanion.
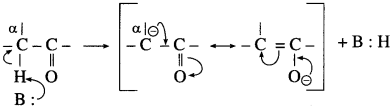
(3) Aldol condensation reaction is characteristic reaction of aldehydes and ketones containing active α-hydrogen atom.
(4) When aldehydes or ketones containing α – H atoms are warmed with a dilute base or dilute acid, two molecules of them undergo self condensation to give β-hydroxy aldehyde (aldol) or β-hydroxy ketone (ketol) respectively. The reaction is known as Aldol addition Reaction.
(5) In aldol condensation, the product is formed by the nucleophilic addition of α-carbon atom of a second molecule which gets attached to carbonyl carbon atom of the first molecule and α-hydrogen atom of the second molecule gets attached to carbonyl oxygen atom of the first molecule forming (- OH) group to give β-hydroxy aldehyde or ketone.
(6) This is a reversible reaction, establishing an equilibrium favouring aldol formation to a greater extent than ketol formation.
(7) For aldehyde :
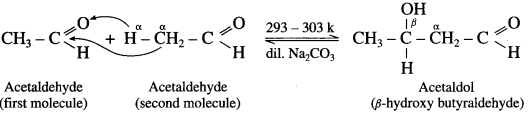
Acetaldol on heating undergoes subsequent elimination of water giving rise to α, β unsaturated aldehyde.
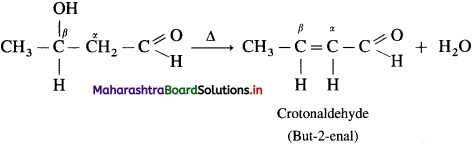
The overall reaction is called aldol condensation. It is a nucleophilic addition-elimination reaction. For ketone :
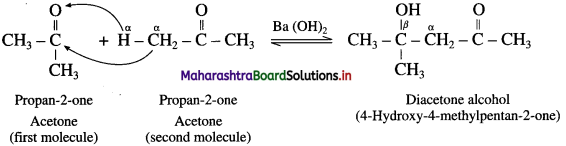
Diacetone alcohol on dehydration by heating forms α, β unsaturated ketone.

![]()
Question ii.
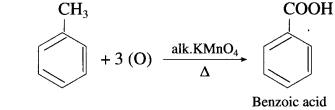
What is the action of the following reagents on toluene ?
a. Alkaline KMnO4, dil. HCl and heat
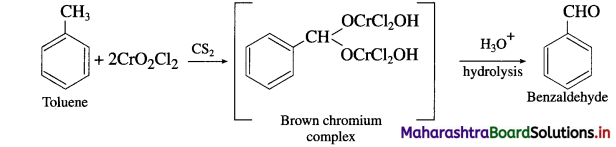
b. CrO2Cl2 in CS2
c. Acetyl chloride in presence of anhydrous AlCl3.
Answer:
(1) Action of alkaline KMnO4 : When toluene is heated with alkaline KMnO4. (methyl group gets oxidised to earboxy lic group) benzoic acid is obtained
(2) Action of CrO2Cl2 in C2:
Answer:
When Loluenc is ircated with soluion of chromyl chloride (CrO2Cl2) in Cs2, brown chromium complex is obtained, which on acid hydrolysis gives benzaldehyde.
(3) Action of acetyl chloride in presence of anhyd. AlCl3.
Answer:
When toluene is treated with acetyl chloride in presence of anhydrous AlCl3 4-methyl acetophenone is obtained.

Question iii.
Write the IUPAC names of the following structures :
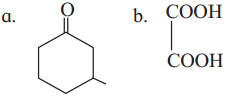
Question iv.
Write reaction showing conversion of p- bromoisopropyl benzene into p-Isopropyl benzoic acid (3 steps).
Answer:

![]()
Question v.
Write reaction showing aldol condensation of cyclohexanone.
Answer:
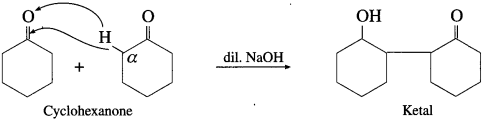
Activity :
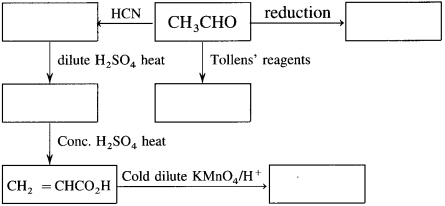
Draw and complete the following reaction scheme which starts with acetaldehyde. In each empty box, write the structural formula of the organic compound that would be formed.
12th Chemistry Digest Chapter 12 Aldehydes, Ketones and Carboxylic Acids Intext Questions and Answers
Use your brain power! (Textbook Page No 254)
Question 1.
Classify the following as aliphatic and aromatic aldehydes.
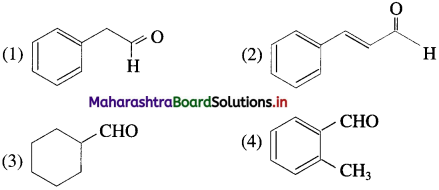
Answer:

Use your brain power! (Textbook Page No 255)
Question 1.
Classify the following as simple and mixed ketones. Benzoplienone, acetoneq hutanoneq acetophenone.
| Compoun | |
| Benzophenone | …………………………………………….. |
| Acetone | …………………………………………….. |
| Butanone | …………………………………………….. |
| Acetophenone | …………………………………………….. |
Answer:
| Compound | |
| Benzophenone | Simple ketone |
| Acetone | Simple ketone |
| Butanone | Mixed ketone |
| Acetophenone | Mixed ketone |
![]()
Use your brain power! (Textbook Page No 264)
Write IUPAC names for the following compounds.

Try this ….. (Textbook Page No 260)
Question 1.
Draw structures for the following :
(1) 2-Methylpentanal
(2) Hexan-2-one
Answer:

Can you tell? (Textbook Page No 260)
Question 1.
Which is the reagent that oxidizes primary alcohols to only aldehydes and does not oxidize aldehydes further into carboxylic acid ?
Answer:
The reagent that is used to make only aldehydes is-heated Cu at 573 K.
Use your brain power! (Textbook Page No 261)
Question 1.
Write the structure of the product formed on Rosenmund reduction of ethanoyl chloride and benzoyl chloride.
Answer:
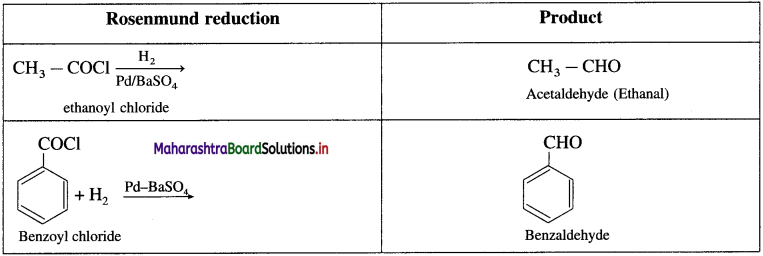
![]()
Can you think? (Textbook Page No 261)
Question 1.
What is the alcohol formed when benzoyl chloride is reduced with pure palladium as the catalyst ?
Answer:
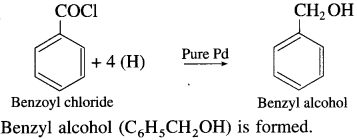
Use your brain power! (Textbook Page No 262)
Question 1.
Name the compounds which are used for the preparation of benzophenone by Friedel-Crafts acylation reaction. Draw their structures.
Answer:
The compounds which are used in preparation of benzophenone by Friedel – Crafts reaction are :
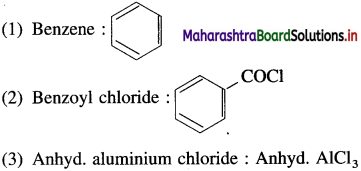
Use your brain power! (Textbook Page No 263)
Identify the reagents necessary to achieve each of the following transformations:
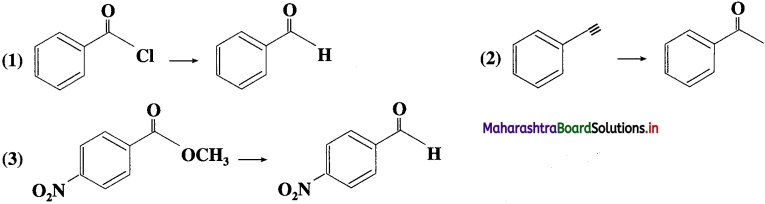
Answer:
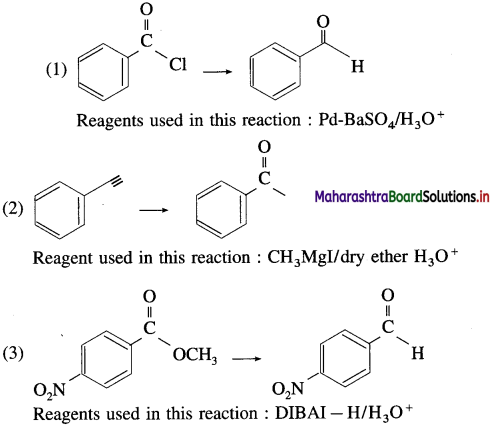
Use your brain power! (TextBook Page No 264)
Predict the products (name and structure) in the following reactions.
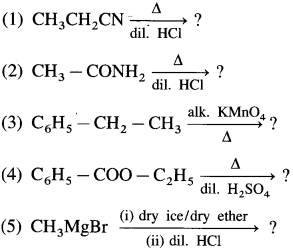
Answer:
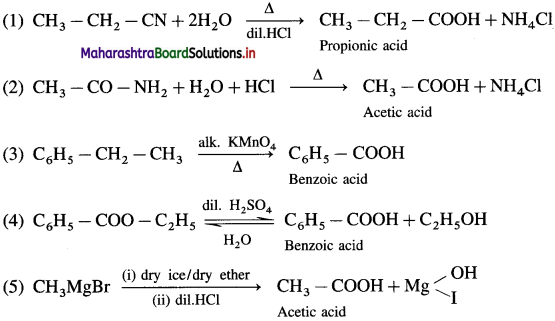
![]()
Problem 12.1 : (Textbook Page No 276)
Question 1.
Alcohols (R – OH), phenols (Ar – OH) and carboxylic acids (R – COOH) can undergo ionization of O – H bond to give away proton H+; yet they have different pKa values, which are 16, 10 and 4.5 respectively. Explain.
Solution :
pKa value is indicative of acid strength. Lower of pKa value stronger the acid. Alcohols, phenols and carboxylic acids, all involve ionization of an O – H bond. But their different pKa values indicate that their acid strengths are different. This is because the resulting conjugate bases are stabilized to different extents.

As the conjugate base of carboxylic acid is best stabilized, among the three, carboxylic acids are strongest and have the lowest pKa value. As conjugate base of alcohols is destabilized, alcohols are weakest acids and have highest pIQ value. As conjugate base of phenols is moderately stabilized, phenols are moderately acidic and have intermediate pBQ value.
Try this….. (Textbook Page No 277)
Question 1.
Compare the following two conjugate bases and answer.
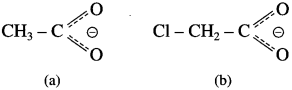
(1) Indicate the inductive effects of CH3 – group in (a) and Cl-group in (b) by putting arrowheads in the middle of appropriate covalent bonds.
(2) Which species is stabilized by inductive effect, (a) or (b) ?
(3) Which species is destabilized by inductive effect, (a) or (b) ?
Answer:
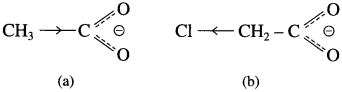
(2) The monochloroacetate ion formed gets stabilised due to electron-withdrawing of Cl atom (- I effect).
(3)  The acetate ion formed gets destabilised due to electron releasing effect of methyl group
The acetate ion formed gets destabilised due to electron releasing effect of methyl group
Use your brain power! (Textbook Page No 277)
Question 1.
(1) Compare the pKa values and arrange the following in an increasing order of acid strength. CI3CCOOH, ClCH2COOH, CH3COOH, Cl2CHCOOH
(2) Draw structures of conjugate bases of monochloroacetic acid and dichloroacetic acid. Which one is more stabilized by – 1 effect?
Answer:
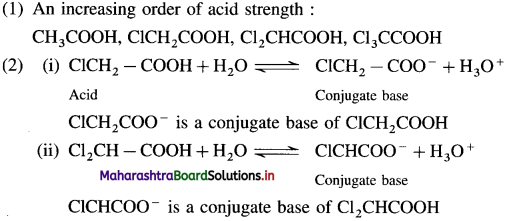
 The dichloroacetate ion formed gets stabilised due electron-withdrawing effect of two chlorine atoms. (- 1 effect)
The dichloroacetate ion formed gets stabilised due electron-withdrawing effect of two chlorine atoms. (- 1 effect)
![]()
Try this….. (Textbook Page No 277)
Question 1.
Arrange the following acids in order of their decreasing acidity.
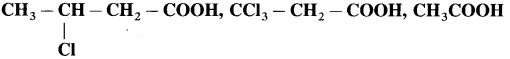
Answer:
Acidity in the decreasing order
Try this ….. (Textbook Page No 267)
Question 1.
Draw the structure of propanone and indicate its polarity.
Answer:

Can you tell? (Textbook Page No 268)
Question 1.
Simple hydrocarbons, ethers, ketones and alcohols do not get oxidized by Tollen’s reagent. Explain, Why?
Answer:
(1) Due to the presence of hydrogen atom in aldehyde group 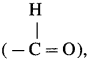 , an aldehyde is oxidised to carboxylic acid
, an aldehyde is oxidised to carboxylic acid  which is not possible in case of ethers, ketones, alcohols and hydrocarbons.
which is not possible in case of ethers, ketones, alcohols and hydrocarbons.
(2) In ketones, carbonyl atom is attached to C-atom, hence C – C bond in  can’t be broken easily.
can’t be broken easily.
(3) H atom attached to carbonyl carbon can be oxidised to – OH group giving carboxylic group  Therefore, aldehyde reduces Tollen’s reagent, whereas simple hydrocarbons, ethers, ketones and alcohols do not reduce Tollen’s reagent.
Therefore, aldehyde reduces Tollen’s reagent, whereas simple hydrocarbons, ethers, ketones and alcohols do not reduce Tollen’s reagent.
Use your brain power! (Textbook Page No 269)
Question 1.
Sodium bisulfite is sodium salt of sulfurous acid, write down its detailed bond structure.
Answer:

Bond structure of sodium bisulfite
Use your brain power! (Textbook Page No 270)
Predict the product of the following reaction:
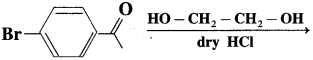
Answer:
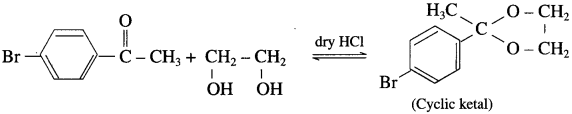
Use your brain power! (Textbook Page No 271)
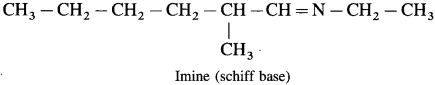
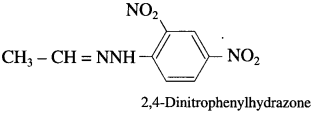
Question 1.
Draw the structures of
(1) The semicarbazone of cyclohexanone
(2) The imine formed in the reaction between 2-methylhexanal and ethyl amine
(3) 2, 4-dinitrophenyl hydrazone of acetaldehyde.
Answer:
(1) The semi carbazone of cyclohexanone.

(2) The imine formed between 2-methyl hexanal and ethyl amine.
(3) 2, 4-dinitrophenylhydrazone of acetaldehyde.
![]()
Try this….. (Textbook Page No 272)
Question 1.
Write chemical reactions taking place when propan-2-ol is treated with iodine and sodium hydroxide.
Answer:

Question 2.
When acetaldehyde Is treated with dilute NaOH, the following reaction is observed.
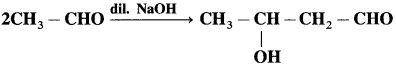
(1) What are the functional groups in the product?
(2) Can another product be formed during the same reaction? (Deduce the answer by doing atomic audit of reactant and product)
(3) Is this an addition reaction or condensation reaction?
Answer:
(1) There arc two functiona’ groups in the product: -CRO and -OH
(2) No other product can be formed in the same reaction.
(3) This is an addition reaction.
Use your brain power! (Textbook Page No 273)
Question 1.
Observe the following reaction.
Answer:
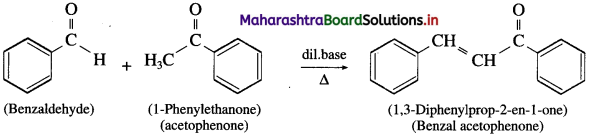
Question 2.
Will this reaction give a mixture of products like a cross aldol reaction ?
Answer:
No, since benzaldehyde, 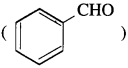 does not have a-hydrogen atom, it will not undergo self aldol condensation.
does not have a-hydrogen atom, it will not undergo self aldol condensation.
Use your brain power! (Textbook Page No 274)
Question 1.
Can isobutyraldehyde undergo a Cannizzaro reaction? Explain.
Answer:
Since isobutyraldehyde contains a-carbon atom, it cannot undergo Cannizzaro reaction.
contains a-carbon atom, it cannot undergo Cannizzaro reaction.
![]()
Can you tell? (Textbook Page No 279)
What is the term used for elimination of water molecule ?
Answer:
Dehydration.
Use your brain power! (Textbook Page No 278)
Question 1.
Fill in the blanks and rewrite the balanced equations.
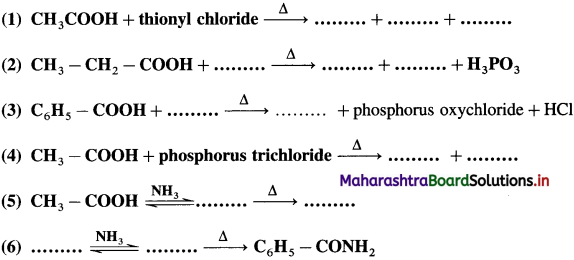
Answer:

Maharashtra State Board 12th Std Chemistry Textbook Solutions
- Coordination Compounds Class 12 Chemistry Textbook Solutions
- Halogen Derivatives Class 12 Chemistry Textbook Solutions
- Alcohols, Phenols and Ethers Class 12 Chemistry Textbook Solutions
- Aldehydes, Ketones and Carboxylic Acids Class 12 Chemistry Textbook Solutions
- Amines Class 12 Chemistry Textbook Solutions
- Biomolecules Class 12 Chemistry Textbook Solutions
- Introduction to Polymer Chemistry Class 12 Chemistry Textbook Solutions
- Green Chemistry and Nanochemistry Class 12 Chemistry Textbook Solutions