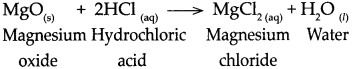
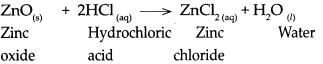
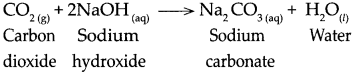
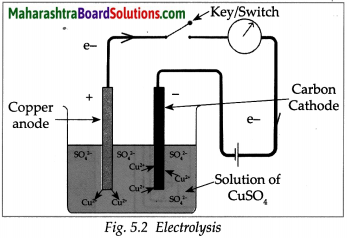
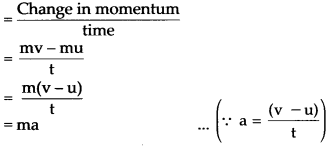
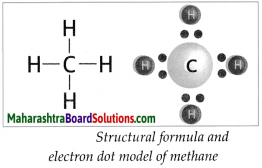
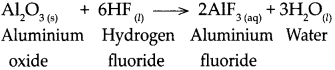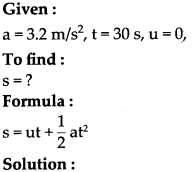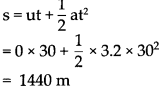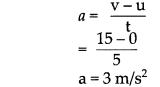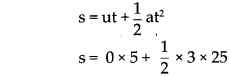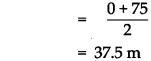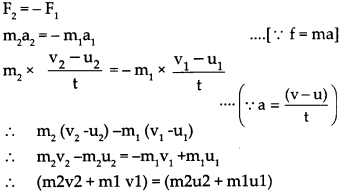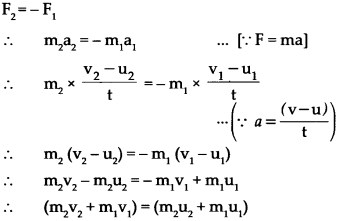Std 10 History Chapter 5 Question Answer Mass Media and History Maharashtra Board
Balbharti Maharashtra State Board Class 10 History Solutions Chapter 5 Mass Media and History Notes, Textbook Exercise Important Questions and Answers.
Class 10 History Chapter 5 Mass Media and History Question Answer Maharashtra Board
History Class 10 Chapter 5 Question Answer Maharashtra Board
Question 1.
(A) Choose the correct option from the given options and complete the statement.
(1) The first English newspaper in India was started by ………………………….. .
(a) James Augustus Hickey
(b) John Marshall
(c) Allen Hume
Answer:
(a) James Augustus Hickey
![]()
(2) Television is an ………………………….. medium.
(a) visual
(b) audio
(c) audio-visual
Answer:
(c) audio-visual
(B) Identify and write the wrong pair in the following set.
(1) ‘Prabhakar’ – Acharya P.K. Atre
(2) ‘Darpan’ – Balshastri Jambhekar
(3) ‘Deenbandhu’ – Krishnarao Bhalekar
(4) ‘Kesari’ – Bal Gangadhar Tilak
Answer:
(1) Wrong Pair: ‘Prabhakar’ – Acharya P.K. Atre
Mass Media And History Class 10 Question 2.
Write short notes :
(1) The role of newspaper in the Indian struggle for independence
Answer:
Newspapers played an important role in the Indian independence struggle. It is as follows
- Newspapers served as an important medium to create awareness during those times.
- They described greatness of Indian culture and history to gather support of masses for the freedom movement.
- They supported social, political and religious movements and opposed imperialism.
- They discussed various social and political issues.
- The ideas of social reformers and various organisations active in independence struggle reached people through newspapers.
(2) Why do we need mass media?
Answer:
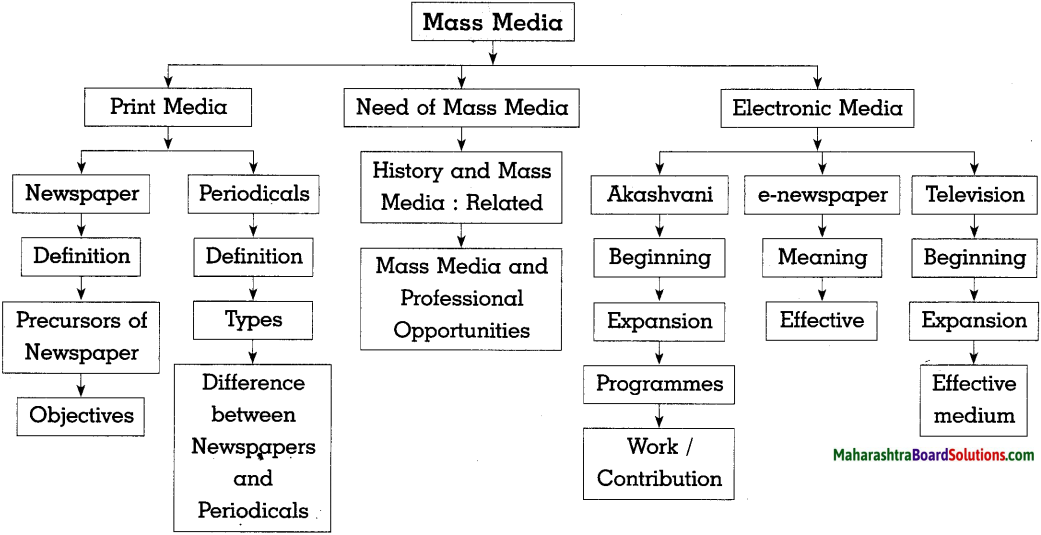
Mass media includes print and electronic and various new media.
- It facilitated free flow of information to all strata of the society and brought the world closer.
- Editorials, various columns and supplements are essential parts of newspapers.
- Readers also get a platform to voice their opinions. In fact, newspapers can help to make democracy stronger.
- Akashrani broadcasted various programmes of the government as well entertainment.
- Awareness creating programmes. It fulfill the need of the government to connect with people.
- Television is an Audio-Visual medium which has made it possible to cross the inherent limitations of newspapers.
- Radio to show the actual visuals of an event to people.
- Mass Media is very important as it plays an important role to strengthen democracy.
(3) Mass Media and professional opportunities.
Answer:
There are many professional opportunities available in printed, electronic and digital media.
- Writers, columnists, editors are required to write articles, columns and editorials in news-papers.
- Newspapers also require reporters to gather news and technicians to work in the press.
- There is requirement of actors and technicians in electronic media.
- Artists are required to present programmes on television, in the same way news presenters, anchors are required.
- If the articles, columns and programmes are based on history, an expert in history is required.
Mass Media And History Class 10 Question Answer Question 3.
Explain the following statements with reasons.
(1) Any information received through mass media needs to be reviewed critically.
Answer:
- Information provided in the media may not represent the exact truth. We need to scan it carefully.
- We have to understand idealistic and investigative motives of newspapers, government policies and prevailing social conditions behiid the newspiece.
- The information received through Mass Media might be prejudiced or give a one-sided idea.
- ‘Stern’, a German weekly magazine, purchased and published a number of so called handwritten diaries of Hitler.
- It then sold them to a number of publication companies.
- However, later it was proved that those diaries were forged. Hence it is essential to verily the information received through Mass Media.
(2) Knowledge of history is essential for newspaper articles.
Answer:
- In order to unfold the background of an event in the news, we have to resort to history.
- Some columns are based on historical events. These columns provide historical information about economical, social and political events in the past.
- Newspapers publish supplements in addition to the regular edition or special issues to mark the completion of 50 or 100 years of an event. On such occasions, one has to review history of that particular event.
- Even while writing columns like what happened in history on this day it is necessary to know past event. Hence, the knowledge of history is essential for writings of such type.
(3) Television is the most popular medium.
Answer:
- Television being an audio-visual medium brings us into contact with events in an exciting and clarifying way.
- It crossed the inherent limitations of newspapers and radio to show actual visuals.
- It becomes possible for people to watch all the national and international events sitting at home.
- Social problems, discussion on education and economics and political events are viewed by people.
- In 1991, Indian government granted permission to private, national and international channels to telecast in India.
- Television became a treasure house of entertainment.
Therefore, the television is the most popular medium.
Class 10 History Chapter 5 Questions And Answers Ssc Board Question 4.
Read the following extract and answer the questions.
Radio: ‘Indian Broadcasting Company’ (IBC), a private radio company was the first one to broadcast daily programmes. Later the same company was taken over by the British Government and named as, ‘Indian State Broadcasting Service (ISBS). On 8th June 1936 it was renamed, as ‘All India Radio (AIR)’.
After Independence, AIR became an integral part of the Ministry of Information and Broadcasting (India). Initially, it broadcasted Governmental programmes and schemes. It was named as ‘Akashvani’ on the suggestion of the famous poet Pandit Narendra Sharma. Akashvani broadcasts various entertainment, awareness creating and literary programmes. It also broadcasts special programmes for farmers, workers, the youth and women. The ‘Vividh Bharati’ programmes are broadcasted in 24 regional languages as well as 146 dialects of Indian languages. Lately, various new channels like ‘Radio Mirchi’ are providing radio services.
(1) Akashavani (AIR) is an integral part of which ministry?
Answer:
- Indian Broadcasting Company, a private radio company was taken over by the British Government in 1927 and named ‘Indian State Broadcasting Service (ISBS)’. On 8th June 1936, it was renamed as ‘All India Radio (AIR)’.
- AIR became integral part of the Ministry of Information and Broadcasting after independence. It was renamed Akashvani on the suggestion of Pandit Narendra Sharma.
- Initially it used to broadcast Government’s programmes and schemes. Later it started broadcasting various entertainment, awareness creating and literary programmes.
- Akashvani started ‘Vividh Bharati1 programmes. It broadcasts special programmes for farmers, workers, the youth and women.
- Vividh Bharati Programmes are broadcast in 24 regional languages and 146 dialects.
(2) What was the new name of IBC?
Answer:
Indian Broadcasting Company (IBC) was taken over by the British Government. It was named as the Indian State Broadcasting Services. (ISBS). On 8th June 1936, it was renamed as ‘All India Radio’ (AIR).
(3) In how many regional languages and local dialects are ‘Vividh Bharati’ programmes broadcasted?
Answer:
People get access to news through social media like Twitter, Instagram, Facebook, YouTube and from web news portals, web channels. This information is available in English and many other languages.
(4) How AIR was named ‘Akashavani’?
Answer:
AIR was named as Akashvani on the suggestion of the famous poet Pandit Narendra Sharma.
Question 5.
Complete the following concept chart.
| Newspapers | Radio | Television | |
| Beginning/Background | |||
| Nature of information/programmes | |||
| Functions |
Answer:
| Newspapers | Radio | Television | |
| Background/Beginning | James Augustus Hickey started Calcutta General Advertiser or Bengal Gazette on 29th January, 1780. It was the first newspaper in English. | A private radio station was started known as Indian Broadcasting Company. | First Doordarshan centre was started in Delhi. |
| Information Programmes | News, articles, columns, opinions of the people, editorials, advertisements etc. are published. | Along with entertainment programmes, have literary, informative programmes on farmers, women and educative values. | Events around the world, movies, music, information about environmental and historical places, sports are shown either live or recorded. |
| Functions | (1) Report daily news (2) Public awareness and mass education. (3) Provide information and strengthen democracy. (4) Oppose injustice and give publicity to developmental work. | (1) Provide news from different sectors. (2) Entertain through music, dramas, songs, etc. (3) Present social problems and educate the masses about it. (4) Conduct discussions on various issues ranging from the environment to culture. | (1) Telecast daily events and entertain. (2) Educate the masses. (3) Publicise programmes which are for social benefit. (4) Bring about social awakening opposing evil traditions and practices. |
Project
Write a review of a historical serial that you have watched.
Memory Map
Question 6.
Complete the sentences by choosing a correct option:
(a) ………………….. is the first newspaper in Marathi.
(a) Deenbandhu
(b) Prabhakar
(c) Darpan
(d) Kesari
Answer:
(c) Darpan
(b) 6th January is celebrated as ………………….. day in Maharashtra.
(a) Periodical Day
(b) Newspaper Day
(c) Printing Day
(d) Journalist Day
Answer:
(d) Journalist Day
(c) The letters ‘Shatpatre1 published in Prabhakar were written by ………………….. .
(a) Lokmanya Tilak
(b) Lokhitvadi
(c) Mahatma Gandhi
(d) Justice Ranade
Answer:
(b) Lokhitvadi.
(d) The honour of printing illustrations for the first time in an Indian newspaper goes to ………………….. .
(a) Dnyanoday
(b) Darpan
(c) Prabhakar
(d) Kesari
Answer:
(a) Dnyanoday
(e) Deenbandhu was started by ………………….. who was a close associate of Mahatma Phule.
(a) Dr. Babasaheb Ambedkar
(b) Lokmanya Tilak %
(c) Narayan Meghaji Lokhande
(d) Krishnarao Bhalekar
Answer:
(d) Krishnarao Bhalekar
(f) ………………….. newspaper was started by Agarkar and Lokmanya Tilak.
(a) Deenbandhu and Induprakash
(b) Darpan and Prabhakar
(c) Dnyanoday and .Digdarshan
(d) Kesari and Maratha
Answer:
(d) Kesari and Maratha
(g) Balshastri Jambhekar started ………………….. the first monthly magazine in Marathi.
(a) Digdarshan
(b) Prabhakar
(c) Darpan
(d) Dnyanoday
Answer:
(a) Digdarshan
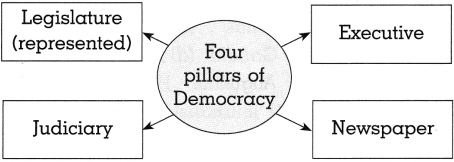
(h) ………………….. was acknowledged as the fourth pillar of democracy.
(a) Representatives
(b) Periodicals
(c) Newspaper
(d) Books
Answer:
(c) Newspaper.
(i) The first English news bulletin was broadcast on 23rd July, 1927 from the …………………… radio station.
(a) Kolkata
(b) Madras
(c) Mumbai
(d) Dblhi
Answer:
(c) Mumbai
(j) Dr. Rajendra Prasad, the first President of India inaugurated the …………………… Doordarshan centre.
(a) Mumbai
(b) Bangalore
(c) Lucknow
(d) Delhi
Answer:
(d) Delhi
(k) Newspapers published special supplements or a special issue to commemorate occasions like completion of seventy-five years of …………………… in 2017.
(a) Khilafat Movement
(b) Non Co-operation Movement
(c) Civil Disobedience Movement
(d) Quit India Movement
Answer:
(d) Quit India Movement
(l) ……………………, a German weekly magazine, had purchased a number of so called handwritten diaries that were later proved forged.
(a) Time Magazine
(b) Statesman
(c) Stern
(d) Reuters
Answer:
(c) Stern
(m) Akashvani has preserved recordings of all speeches delivered by the …………………… on 15th August.
(a) President
(b) Wee President
(c) Prime Minister
(d) Army General
Answer:
(c) Prime Minister
(n) Akashwani comes under the Ministry of …………………… of the Indian Government.
(a) Social welfare
(b) Human Resource and Development
(c) Information and Broadcasting
(d) Education Technology
Answer:
(c) Information and Broadcasting.
Question 7.
Identify the wrong pair in the following and write it:
| Newspaper | Editor |
| (1) Prabhakar (2) Darpan (3) Deenbandhu (4) Kesari | (a) Acharya R K. Atre (b) Balshastri Jambhekar (c) Krishnarao Bhalekar (d) Bal Gangadhar Tilak |
Answer:
Wrong pair: Prabhakar – Acharya R K. Atre
(2)
| Newspaper | Issues |
| (1) Prabhakar (2) Induprakash (3) Deenbandhu (4) Kesari | (a) History of French revolution (b) Advocated widow remarriage (c) Information on Telegraph (d) Voiced social and political problems. |
Answer:
Wrong pair Deenbandhu – Information on Telegraph
(3)
| Newspaper/Magazine/Book | Editor |
| (1) Pragati | (a) Tryambak Shankar Shejwalkar |
| (2) Digdarshan | (b) Narendra Sharma |
| (3) Deenbandhu | (c) Krishnarao Bhalekar |
| (4) Discovery of India | (d) Pandit Nehru |
Answer:
Wrong pair: Digdarshan – Narendra Sharma
Question 8.
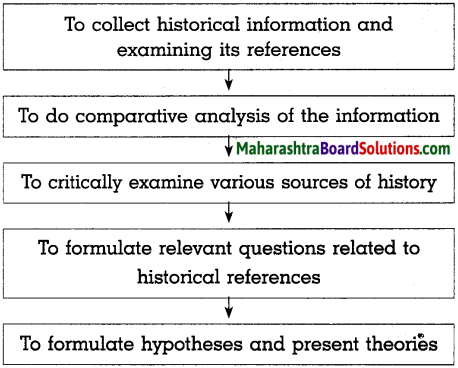
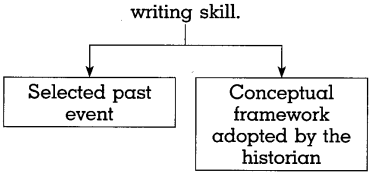
Do as directed:
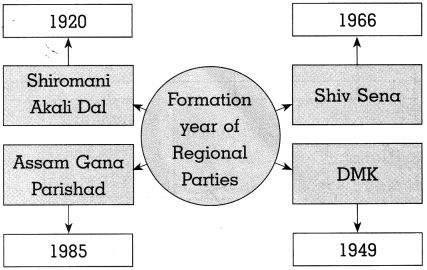
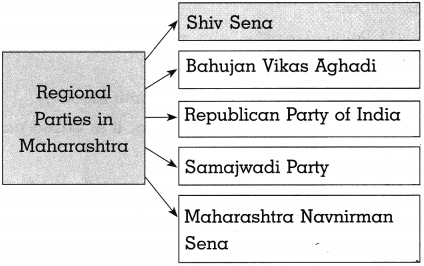
(a) Complete the graphical description
Answer:
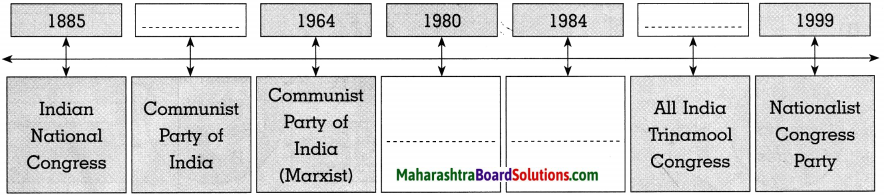
(2)
Answer:
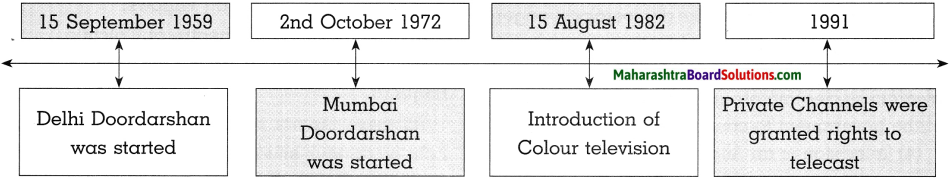
(b) Show the progress of Indian television Time-line:
Answer:
Question 9.
Explain the following concepts:
(a) Electronic or Digital Journalism or Web Journalism.
Answer:
- In the modem times, the computer and internet have become indispensable parts of printing and publishing process. Computer technology has led to the widespread practice of digital journalism.
- Websites run by newspapers are basically extensions of newspapers themselves. Modern periodicals are part of electronic or digital journalism.
- People get access to news through social media like Twitter, Instagram, Facebook, YouTube and from web news portals, web channels. This information is available in English and many other languages.
- Journalists working in this area today have to have many more skills than in the past when writing was the only requirement. Information available on these mediums should be reviewed critically and used with utmost care.
(b) E-newspapers
Answer:
- In recent times, e-newspapers have got prominent place in mass media.
- E-newspaper is not exactly like the printed one. In e-newspapers, news comes in sequence and not based on the nature and the importance of the news, like in printed newspaper e.g.. Front page news. Headline or Last page news.
- The news which we want to read has to be clicked and then it appears on the screen in detail.
- There is space provided for opinion of readers. In 1992, the first edition of the e-newspaper was published by ‘Chicago Tribune1.
- At present, almost all newspapers are available as e-newspapers and people can read them anytime, anywhere using the Internet or computer, tab, laptop or mobile.
- In recent times many newspapers have introduced e-newspapers. The e-newspapers are being received well by the readers.
- Learn to read e-newspapers with the help of your teachers.
Question 10.
Write short notes:
(a) Bengal Gazette:
Answer:
- Bengal Gazette is the first newspaper which was started in India.
- It was started by James Augustus Hickey, an Irish national.
- It was first published on 29th January, .1780. It was also called “Calcutta General Advertiser’.
- Bengal Gazette laid the foundation of newspaper in India.
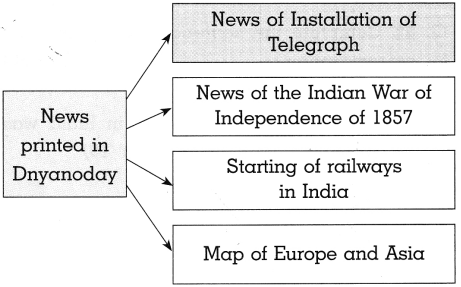
(b) News printed in ‘Darpan’:
Answer:
The ‘Darpan newspaper started by Balshastri Jambhekar printed all types of news like political, economic, social and cultural. Some of them are mentioned below:
- The Accounts of Expenditure from the Three Administrative Divisions of the East India Company.
- The Danger of Russian Attack on the Nation.
- Appointment of a Committee for Cleanliness of the City.
- Remarriage of Hindu Widows.
- The Inception of Theatre at Calcutta.
- Achievements of Raja Ram Mohan Roy in England. All these reports published in the paper throw light on various situations/events of those days.
(c) Television:
Answer:
- The first President of India, Dr. Rajendra Prasad inaugurated Delhi Doordarshan Centre.
- Mumbai Doordarshan started to telecast its programmes on 2nd October, 1972.
- Colour television started on 15th August, 1982. The Indian government granted permission to private, national and international channels in 1991 to telecast in India.
Question 11.
Explain the following sentences with reason:
(a) Newspaper is an important medium of education and information.
Answer:
- Newspapers report events which are interesting to the public. But the importance of newspaper stretches far beyond a passing humari interest in events.
- It covers a miscellany of topical issues. News would involve matters of higher importance like war, global warming, education, national elections or trivial issues such as scandals, gossips and debates on minor controversies.
- Newspapers have contributed significantly to the spread of literacy and the concept of human rights and democratic freedoms.
- They are integral to the development of democracy. In fact, they can help in making the democracy stronger.
- Newspapers not only report the events but continue to shape opinions in the global village.
(b) 6th January is observed as ‘Patrakar Din’ or ‘Journalist Day’ in Maharashtra.
Answer:
- Balshastri Jambhekar started the first newspaper in Marathi on 6th January, 1832 in Mumbai.
- He is referred to as the ‘First Editor’ as he was the first editor.
- He laid the foundation of Marathi newspaper by starting Darpan. As 6th January is his birth date, it is observed as ‘Patrkar Din’ or ‘Journalists’ Day’ in Maharashtra.
(c) Television and history are closely related.
Answer:
- Television plays a major role in developing interest in history. While producing shows and serials based on history and mythology, it is essential to have an accurate knowledge of history and know the minute details.
- ‘Bharat Ek Khoj’, Raja Shivchhatrapati, Ramayana, Mahabharata are among the few popular serials based on history and mythology. While producing these serials.
- It was essential to know the prevalent social conditions, outfits, lifestyle, weaponry, lingual expressions of the people. Historians who had knowledge on these subjects are required.
- While making programmes, based on sportsmen, literature, war, historical events, forts and animal life, it is important to give history of their development in that particular period.
- While conducting discussions on television on topics like social problems, education, economics, health, it is important to give references from the past.
This shows that the knowledge of history is required in the making many of programmes on Television. Hence Television and history are closely related.
Question 12.
Answer the following question in 25-30 words:
(a) Explain the objectives of newspapers.
Answer:
The main objectives of newspapers are as follows:
- Newspapers provide various local, national and international news to the people and inform them about daily events.
- They narrate political, economic, cultural and social history of the country.
- Newspapers fulfill their role as the fourth column of democracy by creating public awareness and becoming a medium of mass education.
- They even condemn the anti-social elements in the society and support the weaker section.
(b) How is history helpful in the planning of the Akashvani programmes ?
Answer:
Akashvani broadcasts all types of programmes from celebration of independence day to entertainment programmes. In planning these programmes, the knowledge of history is essential.
- Akashvani invites historians as experts for discussions while presenting programmes on various occasions such as the anniversaries of births and deaths of national leaders, anniversaries of historical events; speeches of all Prime Ministers/Presidents.
- Programmes like ‘On This Day in History’ is a daily programme which highlights importance of that day and date in history.
- Information has to be verified by historians before it reaches the people. Lectures on the contributions of various national leaders need to be supported by historical information. In the following ways history is helpful in the planning of Akashvani programmes.
(c) How were the message conveyed to the people in olden days?
Answer:
The following were a few means used to convey messages to the people in olden days:
- A town crier would run on the streets beating drums and crying out important news according to the orders of the king.
- So, the news would spread among people by word of mouth.
- Inscriptions with royal decrees were placed at public places.
Question 13.
Read the following passages and answer the questions:”
(a) Which programmes are broadcasted by Akashvani?
Answer:
- Initially, Akashvani broadcasted government programmes and schemes.
- Later it broadcasted various entertainment and literary programmes.
- Akashvani presents various programmes for creating awareness.
- Special programmes are also broadcasted for farmers, workers, youth and women.
(a) On which book is the serial ‘Bharat Ek Khoj’ based on?
Answer:
The Serial Bharat Ek Khoj is based on ‘Discovery of India’, a book written by Pandit Jawaharlal Nehru.
(b) Who directed the serial ‘Bharat Ek Khoj’?
Answer:
The serial was directed by Shyam Benegal.
(c) Which factors/aspects of Indian history are depicted in ‘Bharat Ek Khoj’? OR Why was ‘Bharat Ek Khoj’ a serial telecasted by Doordarshan admired in all parts of India?
Answer:
The television serial ‘Bharat Ek Khoj’ presented social, political and economic life from ancient to the modem period in India.
- It portrayed various aspects of Indian history like Harappan civilisation, Vedic history and the interpretation of epics like Mahabharata and Ramayana.
- It used the technique of dramatisation effectively to recreate the Mauryan period and show the impact of Turk-Afghan invasions.
- The Mughal period and their contributions which have long-lasting effect on social and cultural fabric of India is shown. The rise of Bhakti movement, role of Chhatrapati Shivaji Maharaj in getting swarajya is portrayed.
- The last episodes (finale) of the serial narrate social movements and India’s freedom struggle in modem period.
Thus, the serial effectively portrayed the journey of India from Harappan civilisation to the modern period and therefore was admired in all parts of India.
Question 14.
Answer the following questions in detail:
(a) What were the different means of communication known around the world before the advent of newspaper?
Answer:
The following means of communication were used to convey news before the advent of newspaper:
- Inscriptions with royal decrees placed at public places was a custom in Egypt. Emperor Ashoka followed the sam method to reach out to his subjects.
- In the Roman Empire, roytil decrees were written on papers and those were distribute’d in all regions. It also contained information of various events taking place in the nation and its capital.
- During, the reign of’ Julius Caesar ‘Acta Diurna’, meaning acts of everyday used to be placed at public places in Rome.
- In the 7th century C.E., in China, royal dictates were distributed among people at public places.
- In England handouts were distributed occasionally among people at public places giving information about war or any important events.
- Travellers arriving from different faraway places would add spice to stories from those places and narrate the same to local people. The ambassadors of a king posted at various places would send back important news to the royal court.
(b) Write information on Periodicals based on its types.
Answer:
Magazines and journals which are published at regular intervals are known as Periodicals.
Types:
- They are categorised as weekly, biweekly, monthly, bimonthly, quarterly, six monthly and annual.
- There are some chronicles which are published at no fixed time.
Classification: Periodicals can be classified as popular and scholarly.
- If a periodical aims at specialists and researchers, it is a ‘journal1. Articles are generally written by experts in the subject.
- Popular periodicals are magazines published with variety of content. They can be on fashion, sports, entertainment and films.
- Bharatiya Itihas ani Sanskruti and Marathwada Itihas Parishad Patrika are periodicals of present times. Periodicals are an important source to study history.
(c) Write about the important role of newspaper in the freedom struggle.
Answer:
- The press was the chief instrument for carrying out the political tasks and propagation of nationalist ideology.
- Both English and Vernacular press started by prominent “leaders like Gopal Ganesh Agarkar and Lokmanya Tilak acted as catalyst to the freedom struggle. They started ‘Kesari’ and ‘Maratha’ in 1881.
- Newspapers played a great role in building up an increasingly strong national sentiment and consciousness among people. It was an instrument to arouse, train, mobilise and consolidate nationalist public opinion.
- The newspapers were an effective tool in the hands of social reformers. They exposed social evils such as child marriage, ban on remarriage of the widows, inhuman institution as untouchability, caste fetters, etc. It became a weapon in their hands to educate masses.
- A comparative study was presented in newspaper on western education, knowledge and national education.
- Newspapers also discussed political institutions in India and the west. The main aim of these newspapers was not to gain profit but to serve the people.
(d) Give a short account of the development of Indian television.
Answer:
- Television service started in India in 1959. Dr. Rajendra Prasad, the first Indian President, inaugurated the Delhi Doordarshan centre.
- Mumbai centre began to telecast its programmes on 2nd October 1972. Colour television was introduced in India on 15th August 1982.
- The national telecast began in 1983. Doordarshan started Channels like DD Sports, DD Metro, news, etc. along with 10 regional channels.
- In the year 1991, the Indian government gave permission to private, national and international channels to telecast in India.
- Presently there are more than 800 national and regional channels. Some of them are exclusive news, sports, music, movies and religious channels which telecast programmes 24 hours a day.
(e) Distinguish between Newspapers and Magazines.
Answer:
Newspapers and magazines differ in their format, objectives and duration of getting published. The differences are noted below:
| Newspapers | Magazines |
| 1. Newspapers document the current events. | 1. Magazines give no importance to current news. |
| 2. News, articles, columns, editorials have importance in a newspaper. | 2. Magazines give preferences to particular subject and publish articles on it. |
| 3. Newspapers are also called ‘Dailies’ as they are published every day. | 3. Duration of publication of magazine varies. Some are published weekly, fortnightly, monthly and annually. |
| 4.. The main purpose of newspaper is to report local, national and international news. | 4. Instead of providing news, their content is entertaining and knowledge-based. |
| 5. Newspapers make the people aware of the events happening in the society. They do not stick to any one subject. | 5. Magazines are about a specific topic. On the basis of their appearance, size, readability, content and audience, magazines differ from newspapers. |
| 6. Newspapers mostly write about current news. Whatever happens in the world appears in the newspaper within a span of 24 hours. It shapes public opinion and keep people updated about the activities of the government. | 6, As magazines have lot of detailed information on specific topics they are considered as historical source. |
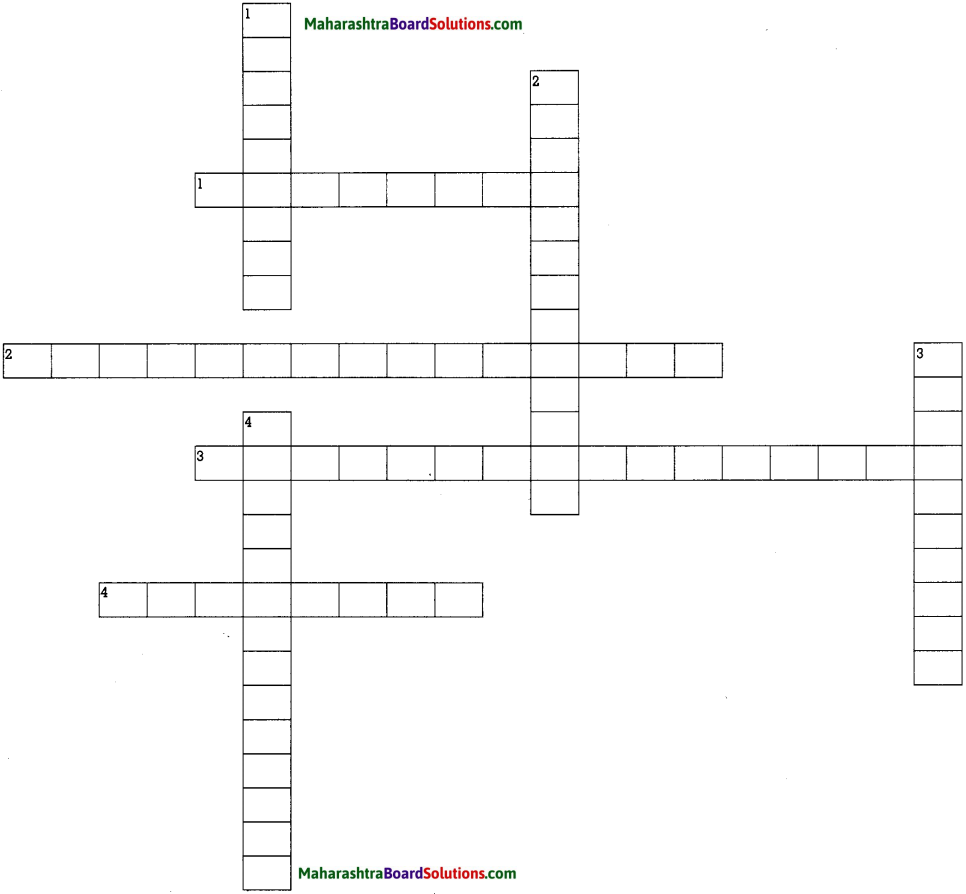
Brain Teaser
Across:
- Referred to as the ‘First Editor’.
- A newspaper representing masses of the Indian society (Bahujan Samaj).
- Tryambak Shankar Shejwalkar edited this journal.
- Letters by Lokhitvadi.
Down:
- The history of French Revolution was published in this newspaper.
- Newspaper started by James Augustus Hickey.
- First monthly magzine in Marathi.
- Pandit Narendra Sharma suggested this name for AIR.
Class 10 History Textbook Solutions Answers
- Historiography Development in the West Class 10 History Textbook Solutions
- Historiography Indian Tradition Class 10 History Textbook Solutions
- Applied History Class 10 History Textbook Solutions
- History of Indian Arts Class 10 History Textbook Solutions
- Mass Media and History Class 10 History Textbook Solutions
- Entertainment and History Class 10 History Textbook Solutions
- Sports and History Class 10 History Textbook Solutions
- Tourism and History Class 10 History Textbook Solutions
- Heritage Management Class 10 History Textbook Solutions