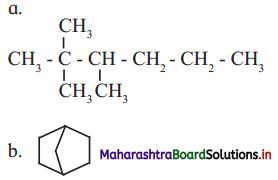
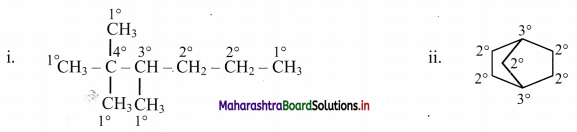
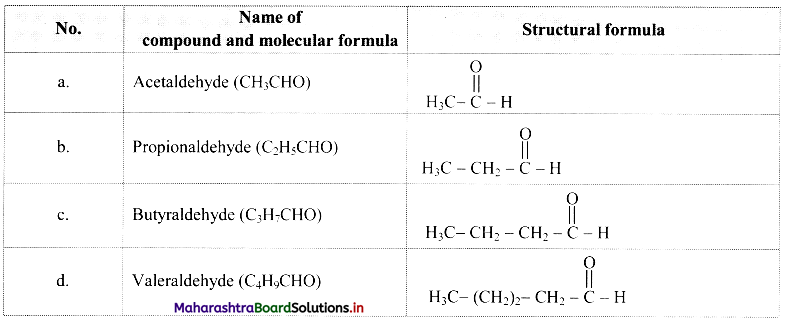
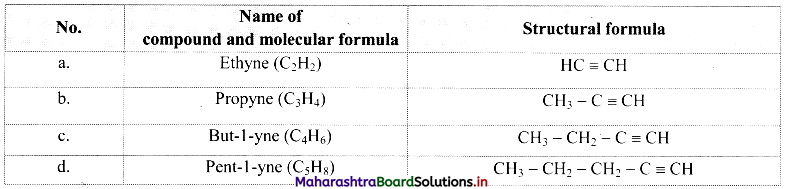
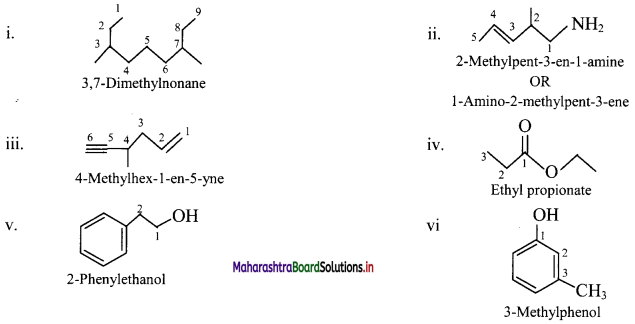
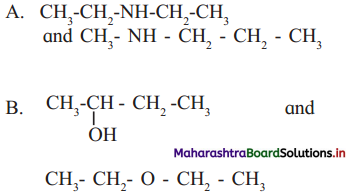
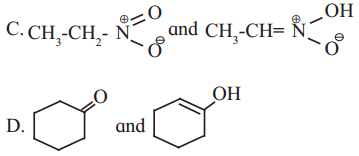
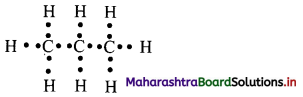
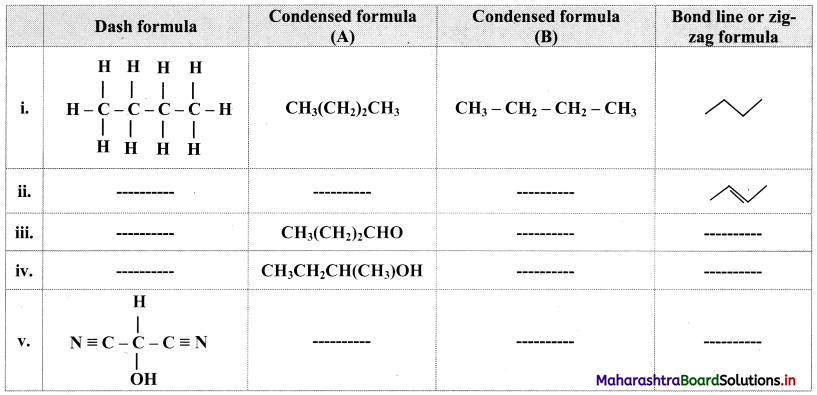
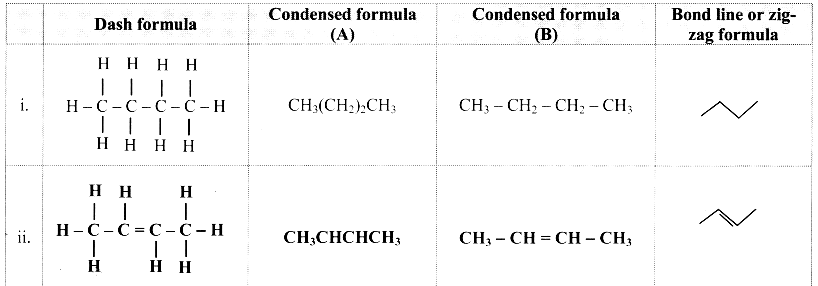
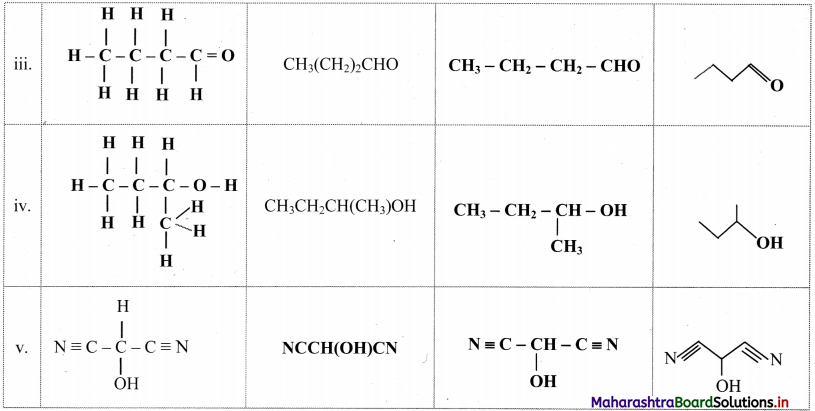
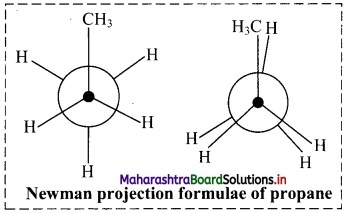
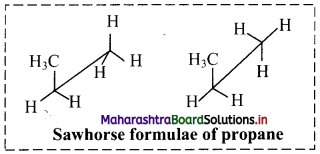
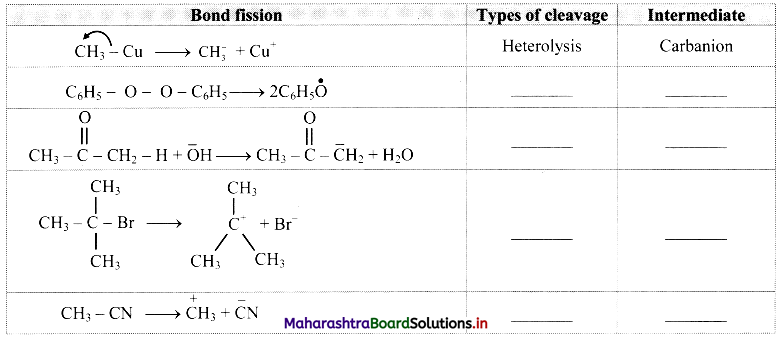
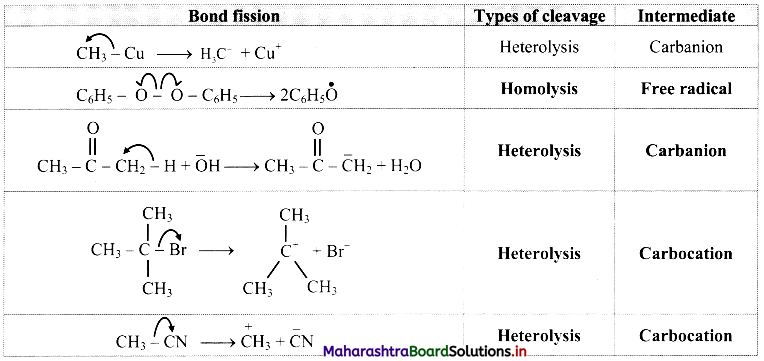
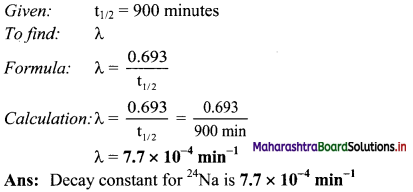
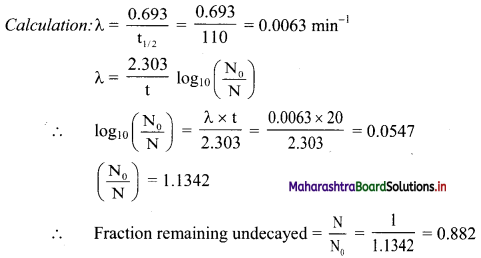
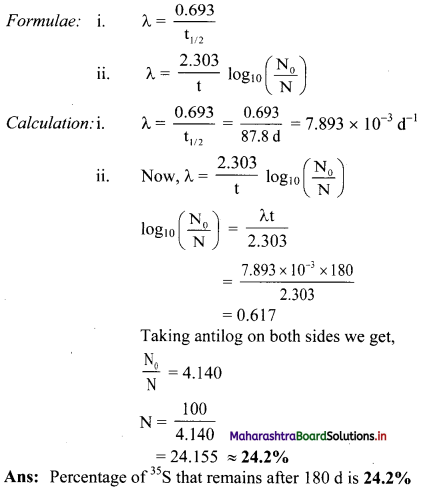
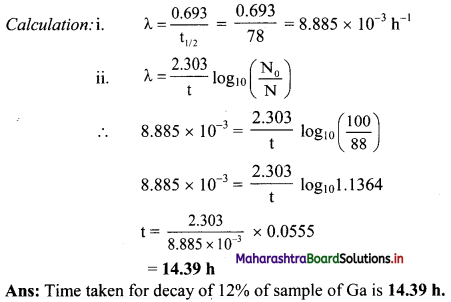
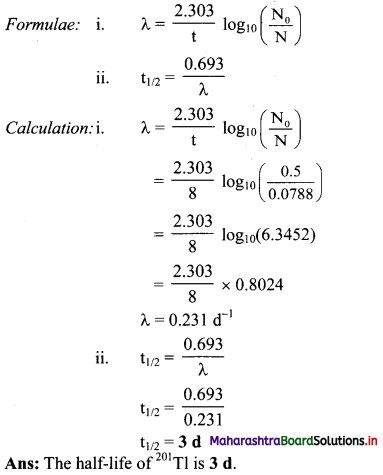
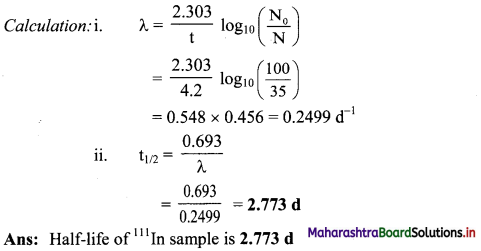
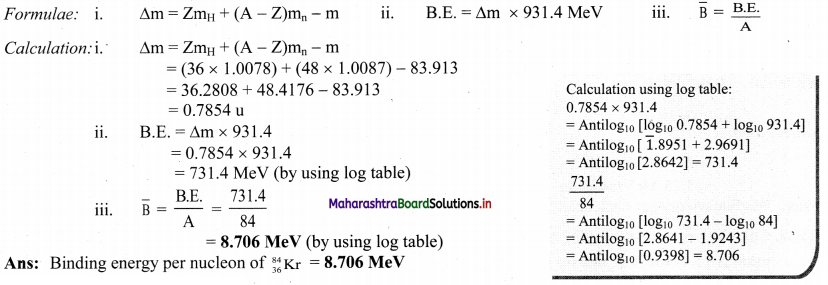
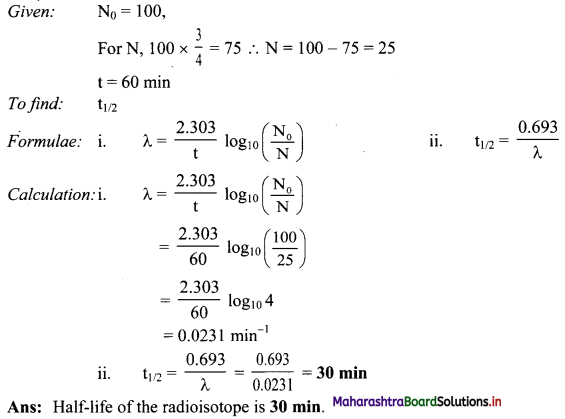
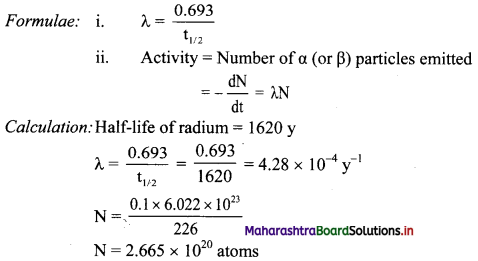
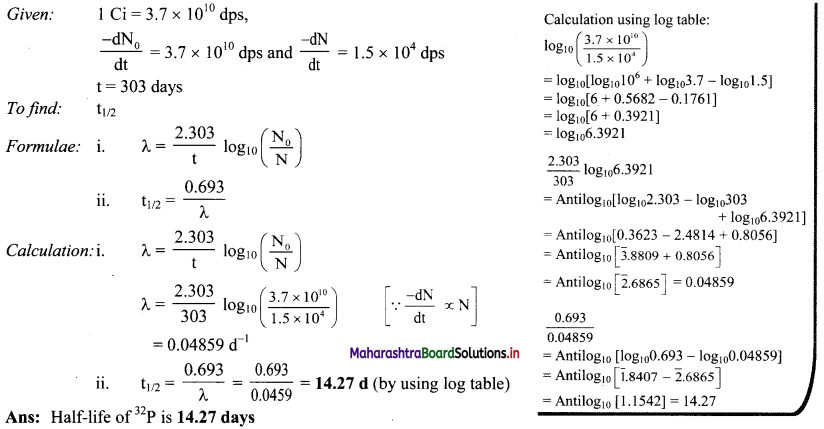
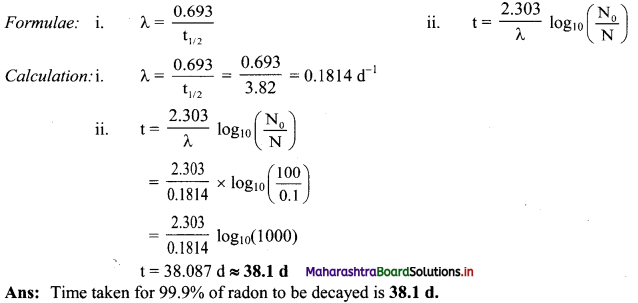
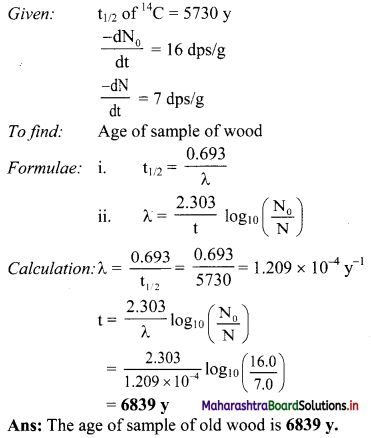
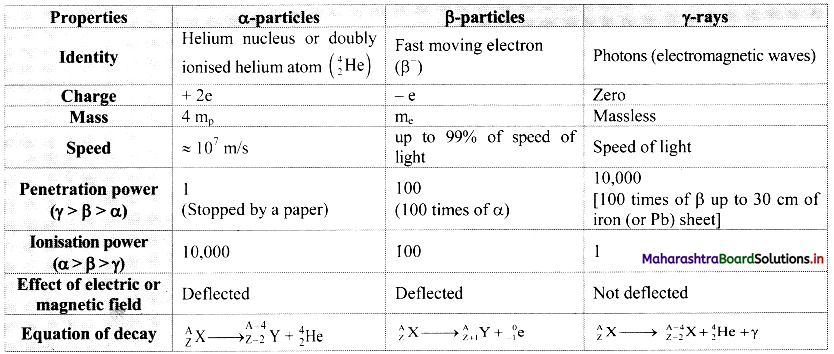
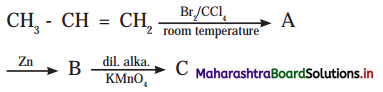
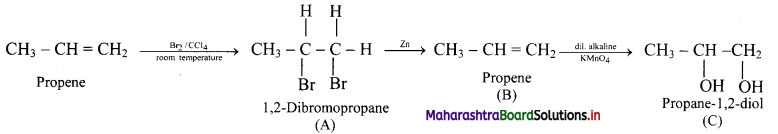
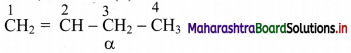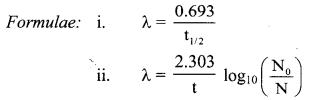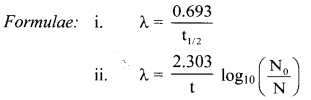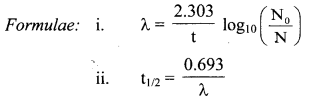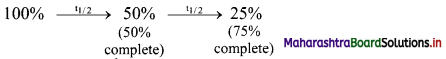Cell Structure and Organization Class 11 Exercise Question Answers Solutions Maharashtra Board
Balbharti Maharashtra State Board 11th Biology Textbook Solutions Chapter 5 Cell Structure and Organization Textbook Exercise Questions and Answers.
Class 11 Biology Chapter 5 Exercise Solutions Maharashtra Board
Biology Class 11 Chapter 5 Exercise Solutions
1. Choose the correct option
Question (A)
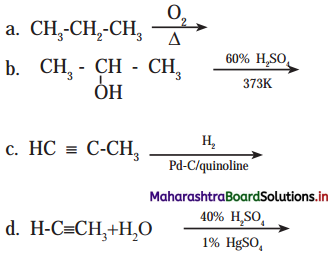
Growth of cell wall during cell elongation takes place by ………….
(a) Apposition
(b) Intussusception
(c) Both a & b
(d) Superposition
![]()
Question (B)
Cell Membrane is composed of
(a) Proteins and cellulose
(b) Proteins and Phospholipid
(c) Proteins and carbohydrates
(d) Proteins, Phospholipid and some carbohydrates
Answer:
(d) Proteins, Phospholipid and some carbohydrates
Question (C)
Plasma membrane is Fluid structure due to presence of
(A) Carbohydrates
(B) Lipid
(C) Glycoprotein
(D) Polysaccharide
Answer:
(B) Lipid
Question (D)
Cell Wall is present in
(a) Plant cell
(b) Prokaryotic cell
(c) Algal cell
(d) All of the above
Answer:
(d) All of the above
Question (E)
Plasma membrane is
(a) Selectively permeable
(b) Permeable
(c) Impermeable
(d) Semipermeable
Answer:
(a) Selectively permeable
![]()
Question (F)
Mitochondria DNA is
(a) Naked
(b) Circular
(c) Double stranded
(d) All of the above
Answer:
(d) All of the above
Question (G)
Which of the following set of organelles contain DNA?
(a) Mitochondria, Peroxysome
(b) Plasma membrane, ribosome
(c) Mitochondria, chloroplast
(d) Chloroplast, dictyosome
Answer:
(c) Mitochondria, chloroplast
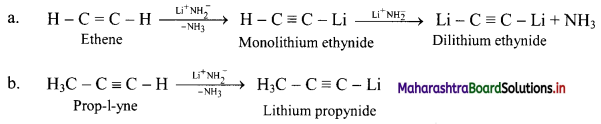
2. Answer the following questions
Question (A)
Plants have no circulatory system? Then how cells manage intercellular transport?
Answer:
1. Plant cells show presence of plasmodesmata which are cytoplasmic bridges between neighbouring cells.
2. This open channel through the cell wall connects the cytoplasm of adjacent plant cells and allows water, small solutes, and some larger molecules to pass between the cells.
In this way, though plants have no circulatory system, plant cells manage intercellular transport.
![]()
Question (B)
Is nucleolus covered by membrane?
Answer:
A nucleolus is specialized structure present in the nucleus which is not covered by the membrane.
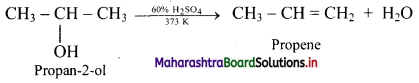
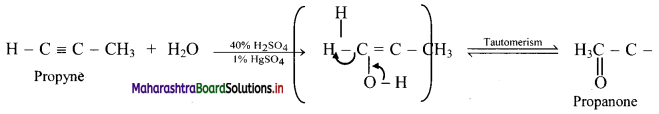
Question (C)
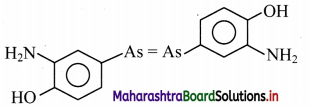
Fluid mosaic model proposed by Singer and Nicolson replaced Sandwich model proposed by Danielli and Davson? Why?
Answer:
- The Davson-Danielli model of the plasma membrane of a cell, was proposed in 1935 by Hugh Davson and James Danielli.
- The model describes a phospholipid bilayer that lies between two layers of globular proteins.
- This model was also known as a Tipo-protein sandwich’, as the lipid layer was sandwiched between two protein layers.
- But through experimental studies membrane proteins were discovered to be insoluble in water (representing hydrophobic surfaces) and varied in size. Such type of proteins would not be able to form an even and continuous layer around the outer surface of a cell membrane.
- In case of Fluid-mosaic model, the experimental evidence from research supports every major hypothesis proposed by Singer and Nicolson.
This hypothesis stated that membrane lipids are arranged in a bilayer; the lipid bilayer is fluid; proteins are suspended individually in the bilayer; and the arrangement of both membrane lipids and proteins is asymmetric. Therefore, Fluid mosaic model proposed by Singer and Nicolson replaced Sandwich model proposed by Danielli and Davson.
Question (D)
The RBC surface normally shows glycoprotein molecules. When determining blood group do they
play any role?
Answer:
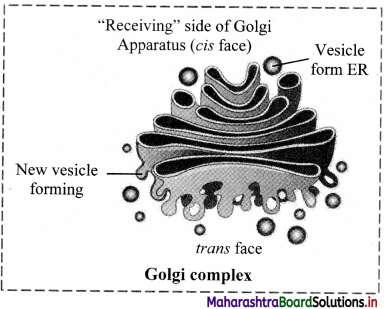
- Glycoproteins are protein molecules modified within the Golgi complex by having a short sugar chain (polysaccharide) attached to them.
- The polysaccharide part of glycoproteins located on the surfaces of red blood cells acts as the antigen responsible for determining the blood group of an individual.
- Different polysaccharide part of glycoproteins act as different type of antigens that determine the blood groups.
- Four types of blood groups A, B, AB, and O are recognized on the basis of presence or absence of these antigens.
![]()
Question (E)
How cytoplasm differs from nucleoplasm in chemical composition?
Answer:
- A thick liquid enclosed by cell membrane which surrounds the central nucleus in eukaryotes or nucleoid region in prokaryotes is known as cytoplasm.
- The cytoplasm shows presence of minerals, sugars, amino acids, t-RNA, nucleotides, vitamins, proteins and enzymes.
- The liquid or semiliquid substance within the nucleus is called the nucleoplasm.
- Nucleoplasm shows presence of various substances like nucleic acid, protein molecules, minerals and salts.
3. Answer the following questions
Question (A)
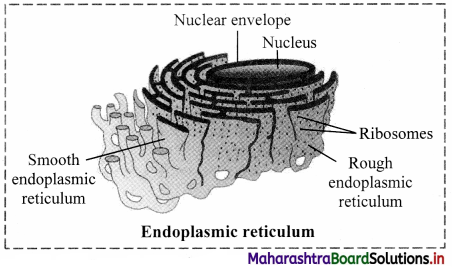
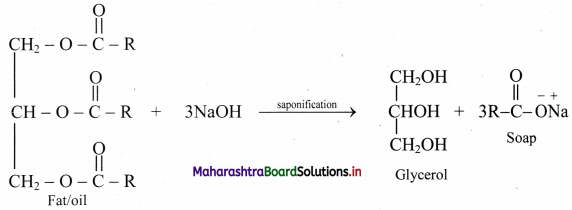
Distinguish between smooth and rough endoplasmic reticulum.
Answer:
Smooth endoplasmic reticulum (SER):
1. Depending on cell type, it helps in synthesis of lipids for e.g. Steroid secreting cells of cortical region of adrenal gland, testes and ovaries.
2. Smooth endoplasmic reticulum plays a role in detoxification in the liver and storage of calcium ions (muscle cells).
Rough Endoplasmic Reticulum (RER):
- Rough ER is primarily involved in protein synthesis. For e.g. Pancreatic cells synthesize the protein insulin in the ER.
- These proteins are secreted by ribosomes attached to rough ER and are called secretory proteins. These proteins get wrapped in membrane that buds off from transitional region of ER. Such membrane bound proteins depart from ER as transport vesicles.
- Rough ER is also involved in formation of membrane for the cell.
The ER membrane grows in place by addition of membrane proteins and phospholipids to its own membrane. Portions of this expanded membrane are transferred to other components of endomembrane system.
Question (B)
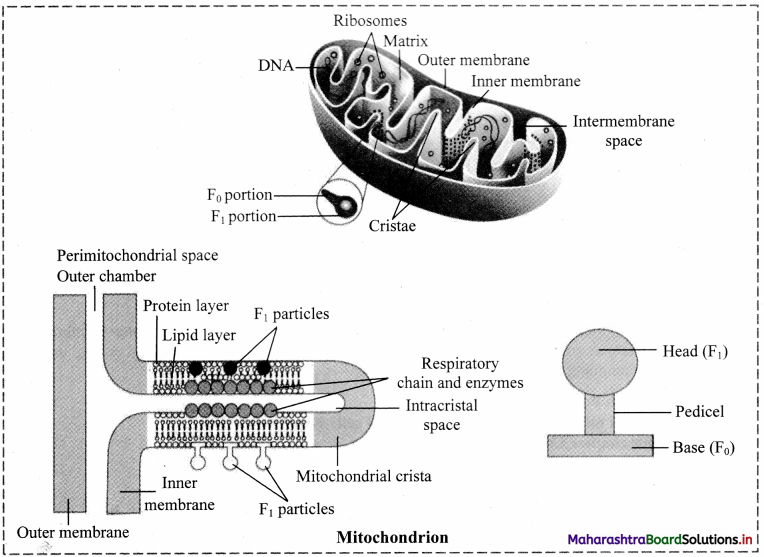
Why do we call mitochondria as power house of cell? Explain in detail.
(Hint: Refer chapter Cellular Respiration.)
OR
Mitochondria are power house of the cell. Give reasons.
Answer:
a. Mitochondria possess oxysomes on its inner membrane. These oxysomes take active part in synthesis of ATP molecules.
b. During cellular respiration, ATP molecules are produced and get accumulated in the mitochondria. They play an important role in cellular activities.
c. only mitochondria can convert pyruvic acid to carbon dioxide and water during cell respiration. Therefore, mitochondria are called ‘power house of the cell’.
![]()
Question (C)
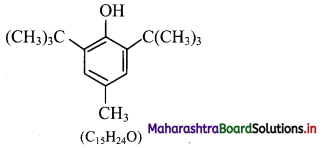
What are the types of plastids?
Answer:
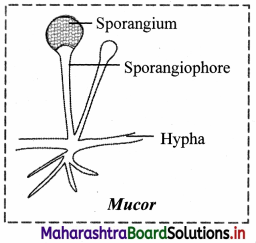
1. Plastids are classified according to the pigments present in it. Three main types of plastids are – leucoplasts, chromoplasts and chloroplasts.
2. Leucoplasts do not contain any photosynthetic pigments they are of various shapes and sizes. These are meant for storage of nutrients:
a. Amyloplasts store starch.
b. Elaioplasts store oils.
c. Aleuroplasts store proteins.
3. Chromoplasts contain pigments like carotene and xanthophyll etc.
a. They impart yellow, orange or red colour to flowers and fruits.
b. These plastids are found in the coloured parts of flowers and fruits.
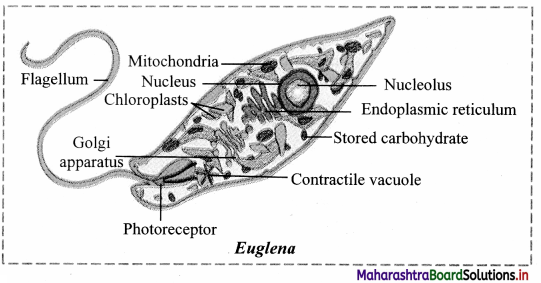
4. Chloroplasts are plastids containing green pigment chlorophyll along with other enzymes that help in production of sugar by photosynthesis. They are present in plants, algae and few protists like Euglena.
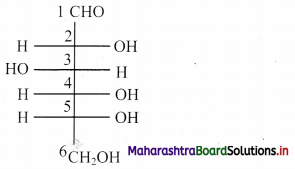
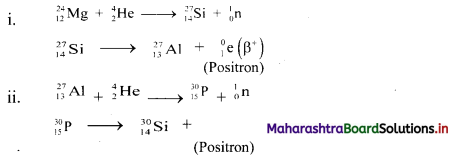
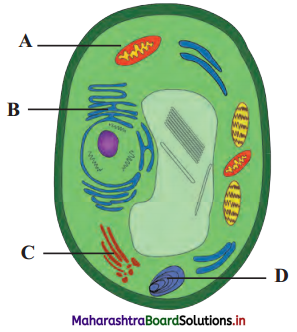
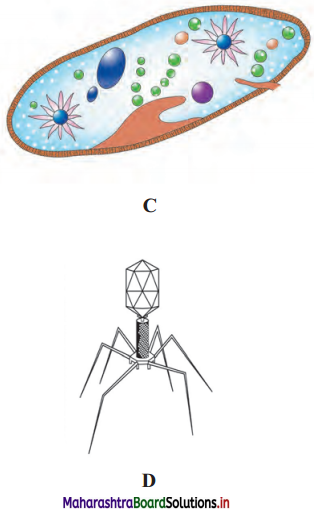
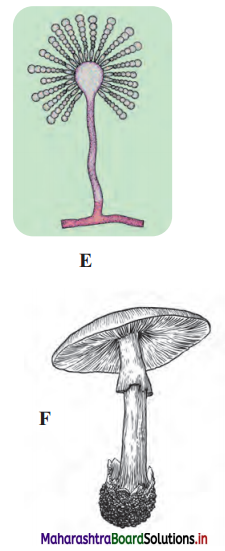
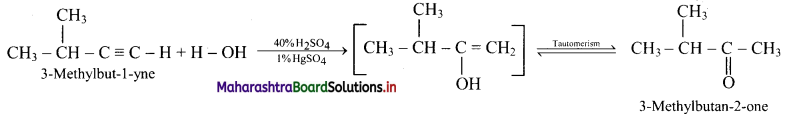
Question 4.
Label the diagrams and write down the details of concept in your words.
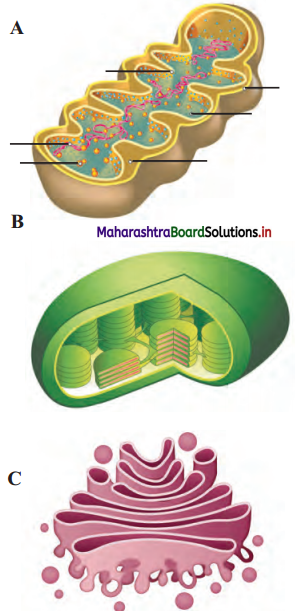
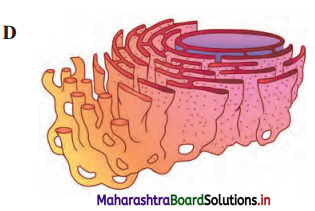
Answer:
A.
B.
![]()
C.
D.
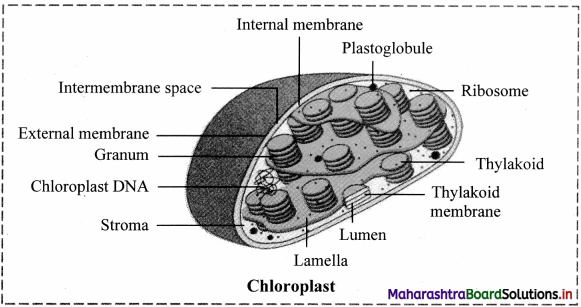
Structure of chloroplast:
- In plants, chloroplast is found mainly in mesophyll of leaf.
- Chloroplast is lens shaped but it can also be oval, spherical, discoid or ribbon like.
- A cell may contain single large chloroplast as in Chlamydomonas or there can be 20 to 40 chloroplasts per cell as seen in mesophyll cells.
- Chloroplasts contain green pigment called chlorophyll along with other enzymes that help in production of sugar by photosynthesis.
- Inner membrane of double membraned chloroplast is comparatively less permeable.
- Inside the cavity of inner membrane, there is another set of membranous sacs called thylakoids.
- Thylakoids are arranged in the form of stacks called grana (singular: granum).
- The grana are connected to each other by means of membranous tubules called stroma lamellae.
- Space outside thylakoids is filled with stroma.
- The stroma and the space inside thylakoids contain various enzymes essential for photosynthesis.
- Stroma of chloroplast contains DNA and ribosomes (70S).
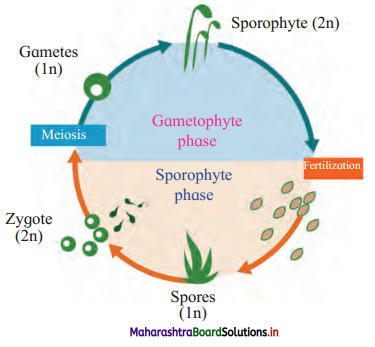
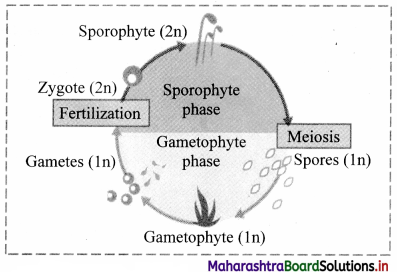
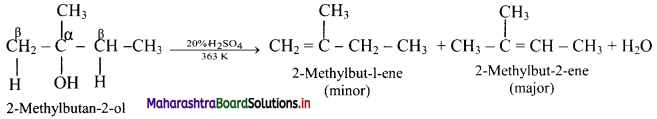
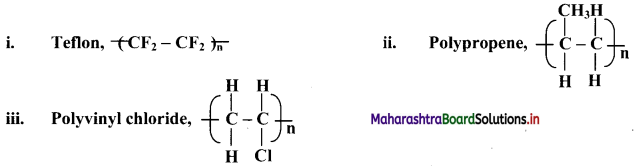
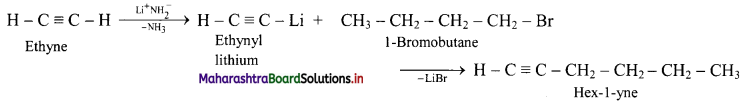
Question 5.
Complete the flow chart.
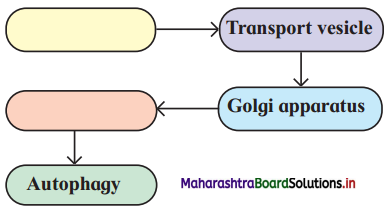
Answer:
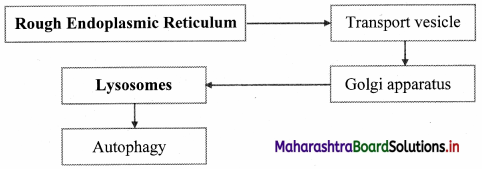
![]()
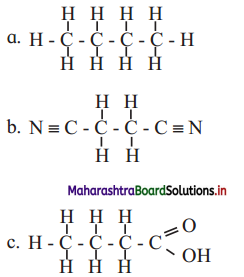
Question 6.
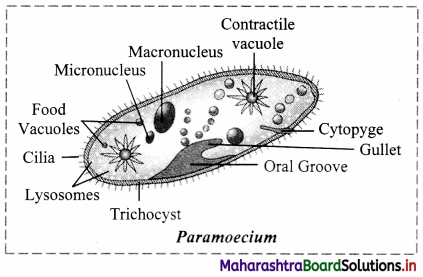
Identify labels A, B, C in the given diagram. Explain how lysosomes perform intracellular and extracellular digestion.
Answer:
1. A: Food vacuole
B: Golgi complex
C: Lysosome
2. Intracellular digestion:
The intracellular digestion is brought about by autophagic vesicle or secondary lysosomes which contain foreign materials brought in by processes like phagocytosis. E.g. Food vacuole in amoeba or macrophages in human blood that engulf and destroy harmful microbes that enter the body.
3. Extracellular digestion:
Extracellular digestion is brought about by release of lysosomal enzymes outside the cell. E.g. acrosome, a cap like structure in human sperm is a modified lysosome which contain various enzymes like Hyaluronidase. These enzymes bring about fertilization by dissolving protective layers of ovum.
Question 7.
Identify each cell structures or organelle from its description below.
- Manufactures ribosomes
- Carries out photosynthesis
- Manufactures ATP in animal and plant cells.
- Selectively permeable.
Answer:
- Nucleolus
- Chloroplast
- Mitochondria
- Plasma membrane
![]()
Question 8.
Onion cells have no chloroplast. How can we tell they are plants?
Answer:
- The bulb of an onion is a modified form of leaves.
- While photosynthesis takes place in the leaves (present above the ground) of an onion containing chloroplast, the little glucose that is produced from this process is converted in to starch (starch granules) and stored in the bulb.
- Starch act as reserved food material in plants.
- Using an iodine solution, we can test for the presence of starch in onion cells. If starch is present, the iodine changes from brown to blue-black or purple. Hence, we can say that though onion cells have no chloroplast they are considered as plants.
Project/ Practical:
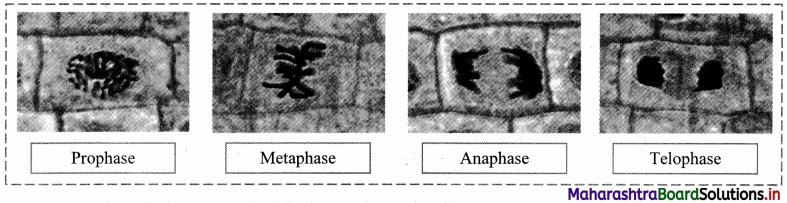
Question 1.
Observe the cells of onion root tip under microscope.
Answer:
The cells of onion root tip will show various stages of cell division when observed under micrscope.
Question 2.
Observe the cells from buccal epithelium stained with Giemsa under microscope.
Answer:
The following observations are made when cells from buccal epithelium stained with Giemsa:
1. Cheek cells are flat and irregular in shape.
2. These cells lack cell wall. A distinct blue nucleus can be observed on viewing the cells under the microscope after Geimsa staining.
11th Biology Digest Chapter 5 Cell Structure and Organization Intext Questions and Answers
Can You Recall? (Textbook Page No. 44)
(i) Who observed cells under the microscope for the first time?
Answer:
Robert Hooke observed cells under the microscope for the first time.
[Note. Cell walls were first observed by Robert Hooke (1665) as he looked through a microscope at dead cells from the bark of an oak tree. But Anton van Leeuwenhoek was first to visualize living cells using a single-lens microscope of his own construction.]
![]()
(ii) Who made the first microscope?
Answer:
The first microscope was made by two Dutch spectacle makers Hans and Zacharias Janssen.
[Note: The Dutch scientist Anton van Leeuwenhoek made microscopes capable of magnifying single-celled organisms in a drop of pond water.]
Find Out (Textbook Page No. 44)
(i) How do a combination of lenses help in higher magnification?
Answer:
a. In a light microscope, visible light is passed through the specimen and then through two glass lenses.
b. The first lens focuses the magnified image of the object on the second lens, which magnifies it again and focuses it on the back of the eye.
c. The glass lenses bend (refract) the light in such a way that the image of the specimen is magnified.
In this way, a combination of lenses helps in higher magnification.
(ii) When do we use plane and concave mirror and diaphragm?
Answer:
a. Concave mirror is used when low-power objective lenses (useful for examining large specimens or many smaller specimens) or high-power objective lenses (useful for observing fine detail) are used, whereas plane mirror is used when oil immersion objective lens is used.
b. The amount of light passing on to the specimen from the condenser (which concentrates and controls the light that passes through the specimen) is regulated by using iris diaphragm.
c. Light is reduced by closing the diaphragm partially for use with dry objectives.
d. Oil immersion objectives require maximum light and this can be achieved by keeping the iris diaphragm fully open.
(iii) What is the difference between magnification and resolution?
Answer:
a. Magnification is the ratio of an object’s image size to its actual size.
b. Resolution is a measure of the clarity of the image; it is the minimum distance two points can be
separated and still be distinguished as separate points.
Can You Recall? (Textbook Page No. 44)
(i) Why bacterial nucleus is said to be primitive?
![]()
(ii) Draw neat and labelled diagram of Prokaryotic cell.
Answer:
1. The DNA-containing central region of bacterial nucleus (prokaryotic cells) i.e. nucleoid, has no nuclear membrane separating it from the cytoplasm. Therefore, bacterial nucleus is said to be primitive.
2. Prokaryotic cell:
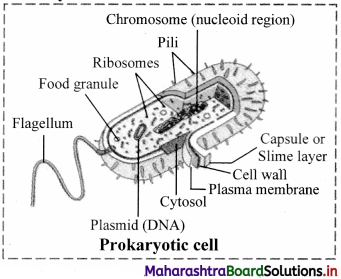
Find Out (Textbook Page No. 46)
Why do basal body of bacterial flagella considered as smallest motor in the world?
Answer:
1. The bacterial flagellum is an organelle for motility made up of three parts:
a. The basal body that spans the cell envelope and works as a rotary motor;
b. The helical fdament that acts as a propeller;
c. The hook that acts as a universal joint connecting these two to transmit motor torque to the propeller.
2. The motor i.e. basal body drives the rotation of the long, helical filamentous propeller at hundreds of hertz to produce thrust that allows bacteria to swim in liquid environments.
Therefore, basal body of bacterial flagella considered as smallest motor in the world.
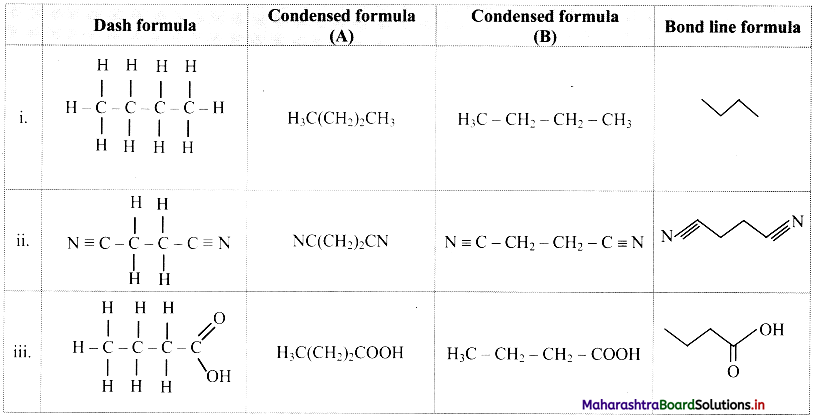
Use your Brainpower (Textbook Page No. 46)
Describe major differences between prokaryotic and eukaryotic cells.
Answer:
| Prokaryotic cell | Eukaryotic cell |
| 1. It is a primitive type of cell. | It is an evolved type of cell. |
| 2. Nuclear membrane is absent. | Nuclear membrane is present. |
| 3. Genetic material is in the form of circular coil of DNA without histone proteins. | Genetic material is in the form of a double helix DNA with histone proteins. |
| 4. Membrane-bound cell organelles are absent. | Membrane-bound cell organelles are present. |
| 5. Plasmids are many in number. | Plasmids are absent. |
| 6. Cytoplasm does not show streaming movement. | Cytoplasm shows streaming movement. |
| 7. Ribosomes are smaller and of 70S type. | Ribosomes are larger and of 80S type. |
| 8. Respiratory enzymes are present on the infoldings of the plasma membrane called mesosomes. | Respiratory enzymes are present within mitochondria. |
| e.g Cyanobacteria (Blue-green algae) and bacteria. | Algae, fungi, plants and animals. |
![]()
Use Your Brain Power! (Textbook Page No. 52)
Are mitochondria present in all eukaryotic cells?
Answer:
a. Mitochondria are found in nearly all eukaryotic cells, including plants, animals, fungi, and most unicellular eukaryotes.
b. Some of the cells have a single large mitochondrion, but frequently a cell has hundreds of mitochondria.
c. The number of mitochondria correlates with the cell’s level of metabolic activity. For e.g. cells that move or contract have proportionally more mitochondria than metabolically less active cells.
d. However, mature red blood cells in humans lack mitochondria.
Can You Recall? (Textbook Page No. 54)
(i) Consider the following cells and comment about the position, shape and number of nuclei in a eukaryotic cell. Add more examples from your previous knowledge about cell and nucleus. Cuboidal epithelial cell, different types of blood corpuscles, skeletal muscle fibre, adipocyte.
Answer:
| Type of cells | Position of nucleus | Shape of Nucleus | Number of nuclei |
| Cuboidal epithelial cell | Central | Round or spherical | 1 |
| Neutrophils | Central | Multilobed/Segmented | 1 |
| Basophils | Central | S Shaped / Twisted | 1 |
| Eosinophils | Central | Bilobed | 1 |
| Monocytes | Central | Kidney Shaped | 1 |
| Lymphocytes | Central | Spherical | 1 |
| Skeletal Muscle Fibre | Peripheral | Oval | Multinucleate |
| Adipocytes | Shifted towards periphery | Eccentric | 1 |
| Simple squamous epithelium | Central | Flat | 1 |
| Ciliated simple columnar epithelium | Near base | Oval | 1 |
(ii) Why nucleus is considered as control unit of a cell?
Answer:
a. Nucleus contains the genetic material of an organism.
b. This genetic material is present in the form of Deoxyribonucleic Acid (DNA) which is responsible for synthesis of various proteins and enzymes.
c. These proteins and enzymes in turn regulate metabolic activities of the cells.
Therefore, nucleus is considered as control unit of a cell.
![]()
(iii) Can cells like Xylem or mature human RBCs called living?
Answer:
a. Xylem is a complex tissue consists of tracheids, vessels, xylem parenchyma and xylem fibres. From these components of xylem, tracheids are dead cells and xylem parenchyma is the only living tissue,
b. RBCs do not possess nuclei once they reach maturity as they have to accommodate haemoglobin in them. They do not require a nucleus to function as they do not reproduce but only serve as a vehicle for the transport of oxygen and carbon dioxide in the blood.
(iv) What is a syncytium and coenocyte?
Answer:
Syncytium: It refers to mass of cells formed by fusion of multiple uninuclear cells and followed by dissolution of the cell membrane.
Coenocyte: It is a multinucleate cell resulted from multiple nuclear divisions without undergoing cytokinesis.
![]()
Can You Recall? (Textbook Page No. 44)
How do onion peel cells and our body cells differ?
Answer:
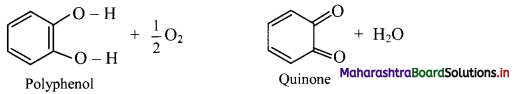
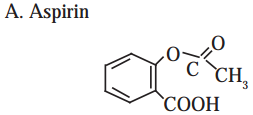
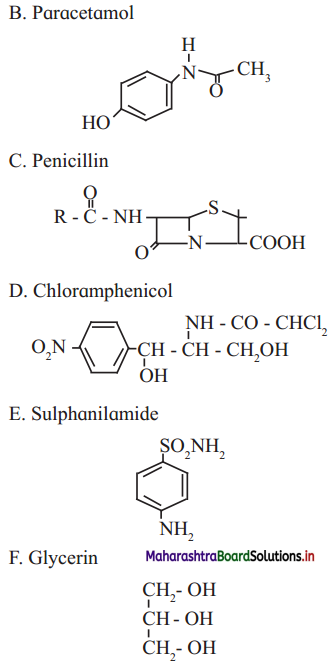
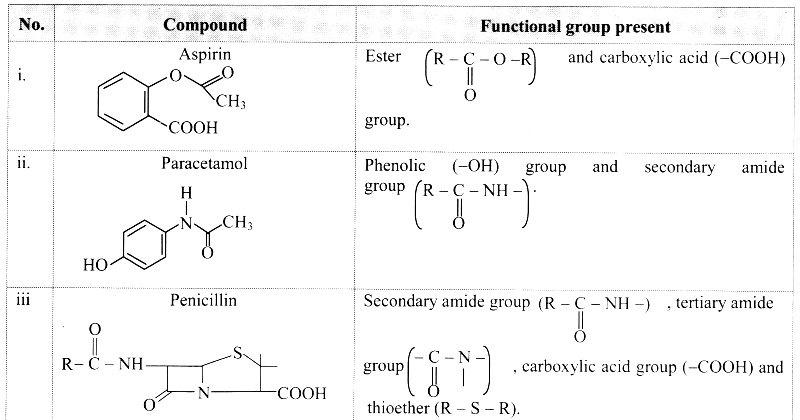
1 – (a, b, d, e,f g)
Maharashtra State Board Class 11 Biology Textbook Solutions
- Living World Class 11 Biology Textbook Solutions
- Systematics of Living Organisms Class 11 Biology Textbook Solutions
- Kingdom Plantae Class 11 Biology Textbook Solutions
- Kingdom Animalia Class 11 Biology Textbook Solutions
- Cell Structure and Organization Class 11 Biology Textbook Solutions
- Biomolecules Class 11 Biology Textbook Solutions
- Cell Division Class 11 Biology Textbook Solutions






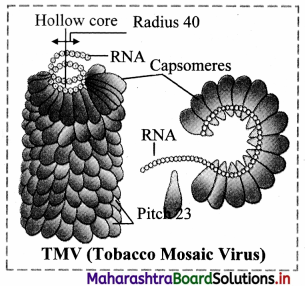
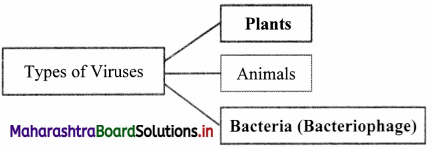
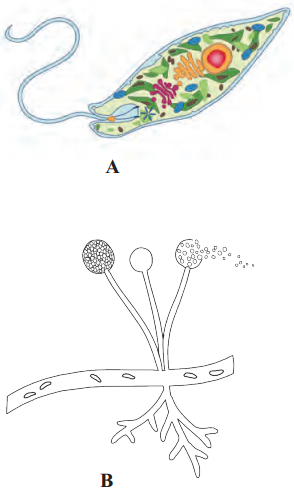
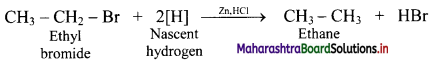
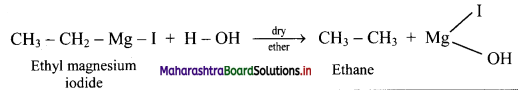
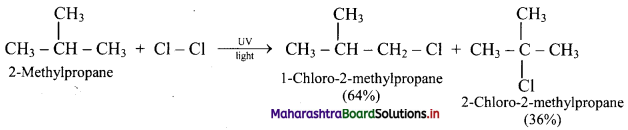
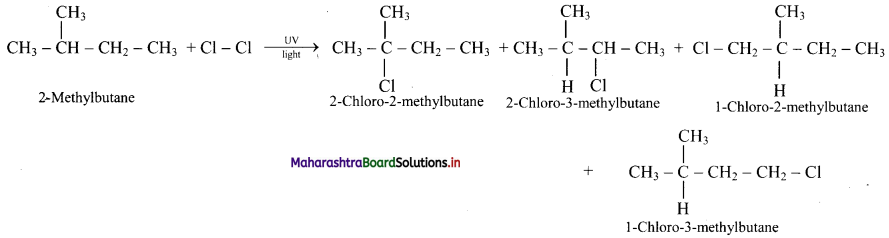
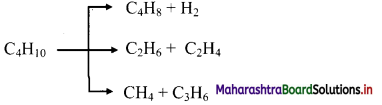
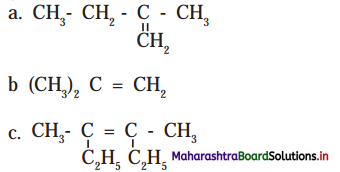
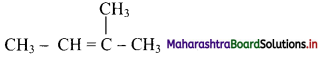
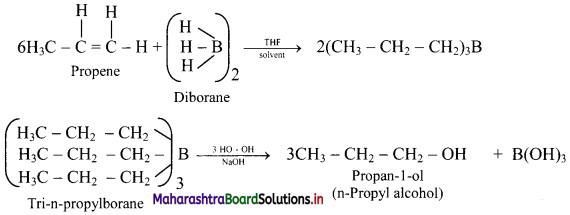
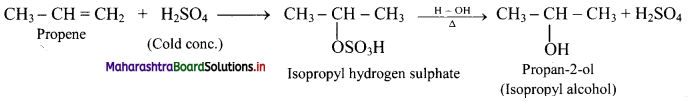
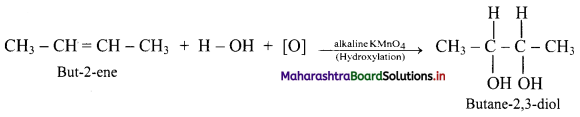



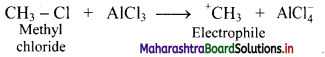
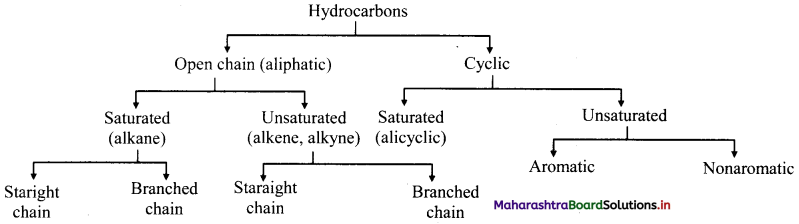
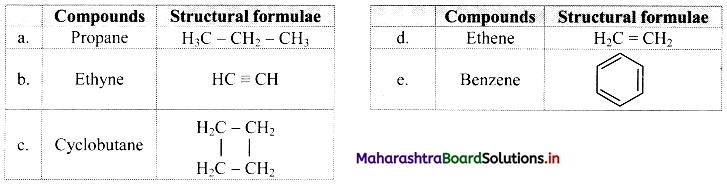
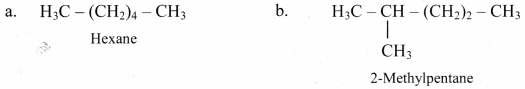
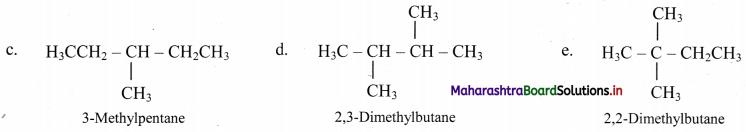
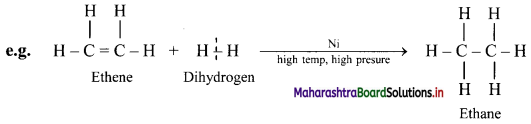

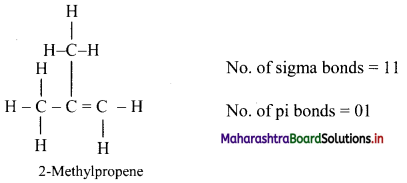
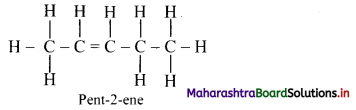
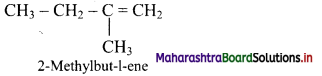
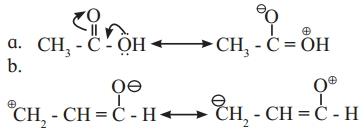
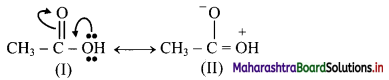
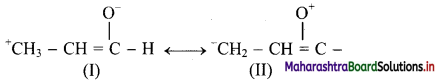
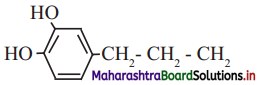
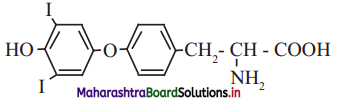
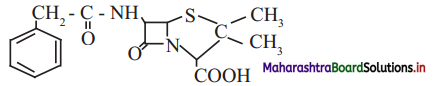
 ,
, ,
,