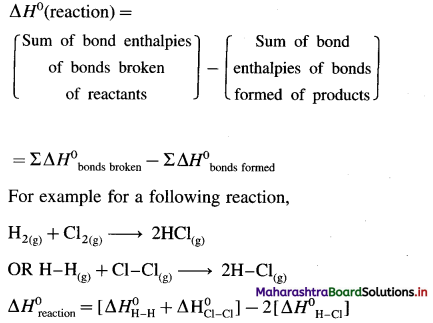
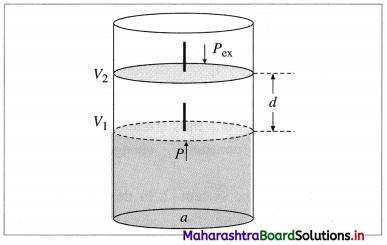
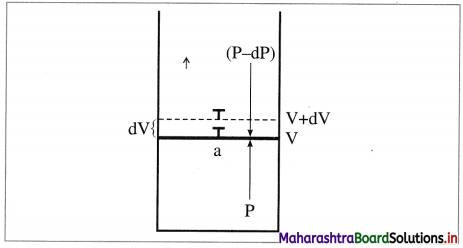
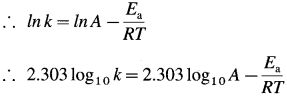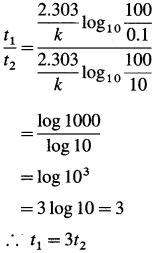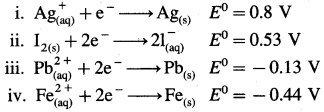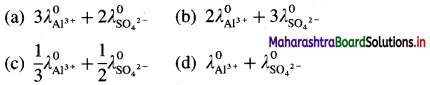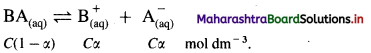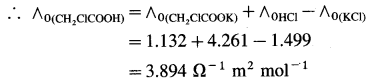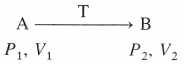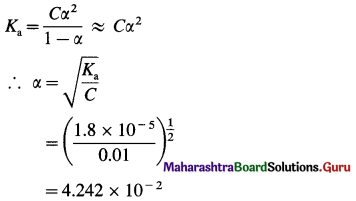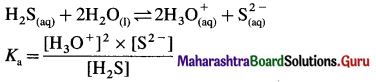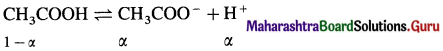Std 12 Political Science Chapter 2 Question Answer Key Concepts and Issues Since 1991: Globalisation Maharashtra Board
Balbharti Maharashtra State Board Class 12 Political Science Solutions Chapter 2 Key Concepts and Issues Since 1991: Globalisation Textbook Exercise Questions and Answers.
Class 12 Political Science Chapter 1 Key Concepts and Issues Since 1991: Globalisation Question Answer Maharashtra Board
Political Science Class 12 Chapter 1 Question Answer Maharashtra Board
1. (A) Choose the correct alternative and complete the following statements
Question 1.
In 1995, GATT was replaced by the
(a) WTO
(b) ECOSOC
(c) UNDP
(d) TRIPS
Answer:
(a) WTO
![]()
Question 2.
……………… refers to a company that operates in several countries but has a distinct home base
(a) Transnational Corporation
(b) Mixed Economy
(c) Multinational Company
(d) Liberalism
Answer:
(c) Multinational Company
(B) Find the odd word.
Question 1.
Mobile, Satellite, Internet, Gramophone.
Answer:
Gramophone (not functioning on modern technology)
(C) State the appropriate concept for the given statements.
Question 1.
The international agency dealing with international trade.
Answer:
World Trade Organization (WTO)
Question 2.
The companies that operate in several countries.
Answer:
Multi National Companies
![]()
(D) Identify the incorrect pair in every set and correct it.
Question 1.
(a) Nestle – Trans National Corporation
(b) Copyrights – Intellectual Property
(c) India – Capitalist Market Economy
Answer:
(c) USA – Capitalist Market Economy
OR India – Economic Liberalism (Mixed economy)
Question 2.
(a) Amnesty International – Human Rights
(b) Green Peace – Environmental Issues
(c) Chernobyl – Trade Agreement
Answer:
(c) Chernobyl – Nuclear disaster
2. State whether the following statements are true or false with reason.
Question 1.
Globalisation brought in the concept of market economy.
Answer:
This statement is True.
(i) During the Cold War, the economic systems followed by countries, depended upon their ideology. For e.g., most West European nations and the USA were free democracies and followed capitalist economy.
(ii) In the era of globalisation there is only ‘market economy’. However, the nature of market economy is determined by the countries ideology for e.g., China has a socialist market economy, West European nations are described as ‘welfare market economies’ and USA is considered as ‘capitalist market economy’.
(iii) In most countries, the State has with draws from economic activities and the private sector and profit motive has propelled the economy.
![]()
Question 2.
Non-state actors have become irrelevant in the age of globalisation.
Answer:
This statement is False.
(i) Good governance and the participatory State focus on the role of the civil society which includes non-state actors such as NGO’s.
(ii) International relations today, are not only between States but also include non-state actors. These sometimes also pose a challenge to the position of the State. Globalisation has made non-state actors relevant. This includes organisations which are beneficial e.g., NGO’s working for humanitarian issues as well as threatening organisations e.g., terrorist outfits.
3. Explain the correlation between the following.
Question 1.
Globalisation and culture
Answer:
Globalisation refers to the rapid spread of goods and services, technology and information, ideas and culture, trade and interactions across the world. It is the connection of different parts of the world resulting in the expansion of international cultural, informational, economic and political activities. Events in one part of the world have an impact on other parts of the world. Changes have taken place economically and culturally.
Today a ‘global cosmopolitan culture’ has emerged i.e movement of people across the world and public awareness of global issues. This is noticed in matters like values eg secularism, clothing food choices, ways of celebrating festivals, etc. There is international awareness of India’s rich cultural and historical heritage. Similarly, westernisation and urbanisation have influenced Indian society eg breakup of the traditional joint family and rise of individualism and materialism in the country.
![]()
Question 2.
GATT and WTO
Answer:
The General Agreement on Tariffs and Trade (GATT) was signed on 30th October 1947 by 23 countries with the purpose to promote international trade by reducing/eliminating trade barriers such as tariffs or quotas. It came into force on 1st January 1948. It aimed to boost economic recovery after World War II through reconstructing and liberalizing global trade. It introduced the most favoured nation principle. GATT was refined over 8 rounds of negotiations, leading to creation of World Trade Organization (WTO) which replaced GATT on 1st January 1995.
WTO covers services and intellectual property also. It is the international agency overseeing the rules of international trade i.e., it promotes free trade agreements, organizes trade negotiations, settles trade disputes, etc. It’s headquarters is in Geneva. It has 123 member States. The WTO dispute settlement system is faster, more automatic than the GATT system and it’s rulings cannot be blocked.
4. Express your opinion of the following.
Question 1.
Participatory State is beneficial to the society.
Answer:
Participatory State advocates more involved forms of citizen participation and greater political representation than traditional representative democracy. It goes beyond traditional democratic practices wherein decisions are made by the majority. In a participatory State, all sections of the society are involved in the making of policy. Participatory State is beneficial as it gives citizens a central role in public policy through public discussion, negotiations, voting, etc. It emphasizes the importance of making citizens aware and providing for a form of communication which promotes political dialogue.
![]()
5. Answer the following question in 80 to 100 words.
Question 1.
What are the positive and negative aspects of Globalisation?
Answer:
Globalisation refers to the rapid spread of goods and services, technology and information, ideas and culture, trade and interactions across the world. It is the connection of different parts of the world resulting in the expansion of international cultural, informational, economic and political activities. In the early 1990s, the term globalisation was used to include economic, political, socio¬cultural, technological and ideological changes that occurred in the world in the post cold war era. The world has become more interconnected due to advances in technology and communication. Events in one part of the world have an impact on other parts of the world. Changes have taken place economically and culturally.
The Positive aspects of globalisation are-
- It creates more employment opportunities.
- It encourages free trade.
- It leads to better choice of goods and services to the consumer.
- It leads to wider investments in developing countries.
- It enhances efficiency of the tertiary sector i.e., banking and finance.
- It increases purchasing power of citizens and enhances their standard of living.
- It increases labour productivity and reduces capital-output ratio.
- It helps to increase efficiency in the production system.
The negative aspects of globalisation are-
- Globalization promotes technological adaption to increase productivity but has also resulted in loss of jobs.
- Local/small scale industries cannot withstand competition from the MNC’s and may be bought off or shut down.
- Less developed countries may become dependent on the technologically superior countries.
- It has caused specialization of labour and so there are few employment opportunities for unskilled labour.
- It has led to increased gap between rich and poor nations.
- It may lead to overexploitation of resources and negatively impact the environment.
- It leads to the harmful effects of consumerism.
- It may lead to reduction in social welfare schemes in both developed and developing countries.
Activity
Talk to people of the older generation to find out what changes have taken place in the age of globalisation.
![]()
Class 12 Political Science Chapter 2 Key Concepts and Issues Since 1991: Globalisation Intext Questions and Answers
Activity (Text Book Page No. 18)
Question 1.
What has been the impact of globalisation on the Indian agricultural sector, especially the small farmer?
Answer:
Globalisation has both positive and negative consequences on Indian agriculture.
The positive consequences are-
(i) Availability of modern agro technologies in pesticides / herbicides, fertilizers, new varieties of high yield seeds to increase food production.
(ii) There are new markets for agricultural products.
(iii) Farmers can sell their goods directly to companies and eliminate the role of middlemen.
The negative effects of globalisation on agriculture are-
- Farmers are shifting from traditional / mixed cropping to unsustainable cropping practices mainly for cash crops.
- MNC’s have captured the India market, making farmers dependent on expensive HYV seeds, fertilizers, etc.
- Small and marginal farmers may not be able to avail of the advantages of globalisation. They may be pushed into debt leading to tragic consequences like farmer suicides.
![]()
Question 2.
Find out what the Arab Spring movement was and how social networking was used during that movement. (Text Book Page No. 21)
Answer:
Arab Spring was a series of protests and uprisings against the governments that spread across large parts of the Arab world in the early 2010s. (i.e. December 2010 to December 2012). It began with protests in Tunisia and spread quickly to other countries like Libya, Egypt, Yemen, Syria and Bahrain. There were riots, civil wars and the main slogan of protestors was “the people want to bring down the regime”.
There were sustained street demonstrations in Iraq, Algeria, Morocco, Jordan, Lebanon, etc. The social media i.e. facebook, etc. was the driving force behind the swift spread of the revolutions. The results of these movements were that regimes of Tunisia (Abidine Ben Ali), Egypt (Hosni Mubarak) Libya (Gaddafi), Yemen (Abdullah Saleh) were ousted while in Syria, Iraq, etc., a full scale civil war resulted. Only in Tunisia, there was a transition to constitutional democratic government.
Question 3.
Find out cases where agitations have used social networking to highlight their demands. (Text Book Page No. 21)
Answer:
Social networking and micro media have aided many protests and agitations. Some examples are:
(i) Arab Spring movements (2010-2012) used media power eg., Facebook to over throw despotic rulers e.g., Gaddafi in Libya or Hosni Mubarak in Egypt.
(ii) In India, the Anti-Corruption Movement led by Anna Hazare (2011) was helped by extensive media coverage and social media posts specially among the youth and students.
(iii) Social networking played a vital role in the “Me Too” movement all over the world to expose workplace sexual harassment especially in the glamour industry.
(iv) Social networking played a major role in galvanising support during the pro-democracy demonstrations in Hong Kong.
(v) Various social media handles fuelled the protests against NRC, CAA, etc., in various States of India.
![]()
Question 4.
Can the cooperative movement of India be an answer to the domination of multinational and transnational companies? The philosophy of the cooperative movement is to provide both, empowerment and finance to the members while that of the corporations work on profit motive. Give your opinion on this. (Text Book Page No. 17)
Answer:
Cooperative Movement in India can be traced to the Cooperative Credit Societies Act (1904). India’s first Prime Minister, Jawaharlal Nehru had strong faith in the cooperative movement. Hence, cooperatives became an integral part of Five Year Plans in India. In 1958, National Development council recommended setting up of Cooperative Marketing Societies. The major sectors where cooperatives dominate are in dairy, agriculture, banking and rural credit, etc. Article 43, Part IV (DPSP) of the constitution, mentions about promotion of cooperatives mainly in rural areas.
The importance of the cooperative sector.
- it provides agricultural credits where the State and private sectors have not been able to do so.
- it helps to overcome the constraints of agricultural development.
- it provides empowerment to the members.
- it can be an answer to the domination by the MNC’s which work solely on the profit motive. If the problems of cooperatives are overcome, they can strengthen the financial sector and lessen our reliance on MNC’s.
Maharashtra State Board 12th Std Political Science Textbook Solutions
- The World Since 1991 Class 12 Political Science Solutions
- Key Concepts and Issues Since 1991: Globalisation Class 12 Political Science Solutions
- Key Concepts and Issues Since 1991: Humanitarian Issues Class 12 Political Science Solutions
- Contemporary India: Challenges to Peace, Stability and National Integration Class 12 Political Science Solutions
- Contemporary India: Good Governance Class 12 Political Science Solutions
- India and the World Class 12 Political Science Solutions

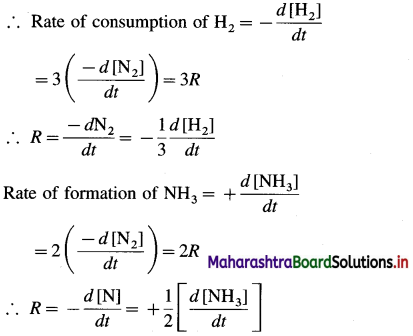
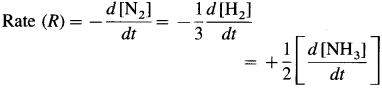

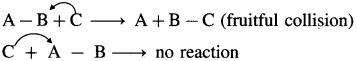
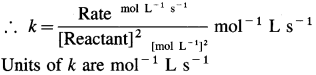
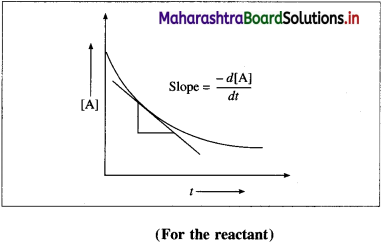
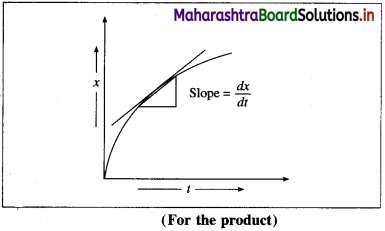
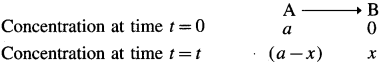
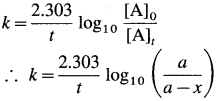

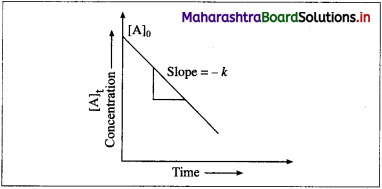

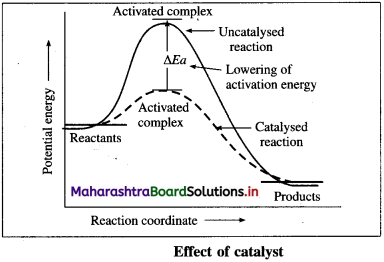
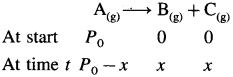
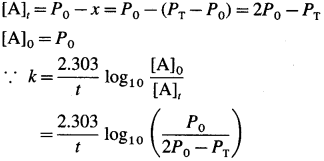

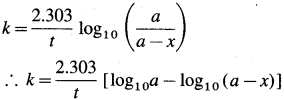


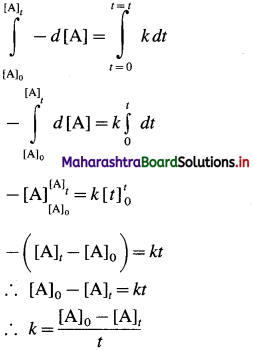
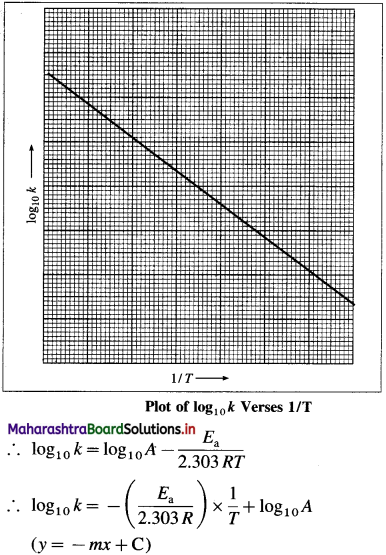
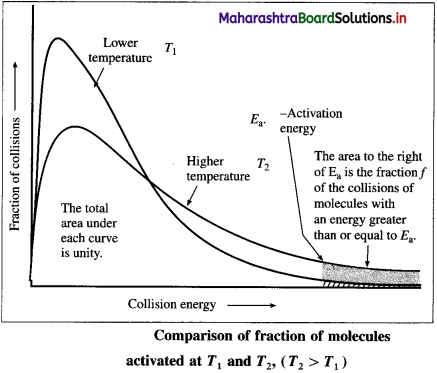
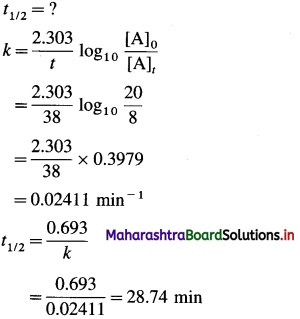
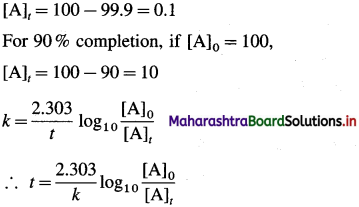
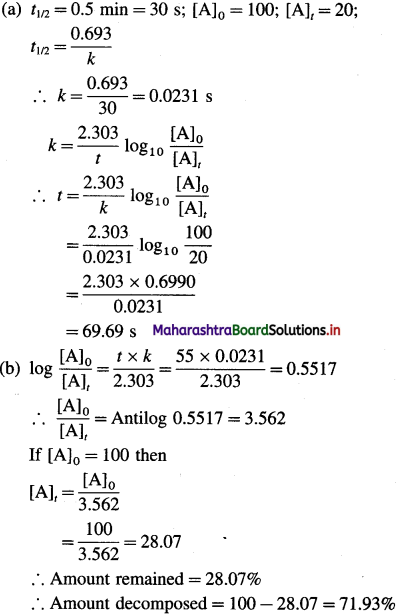
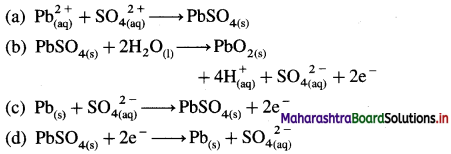
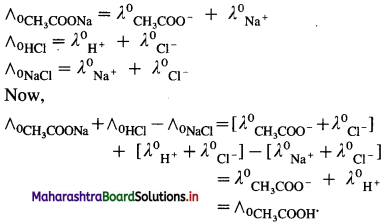
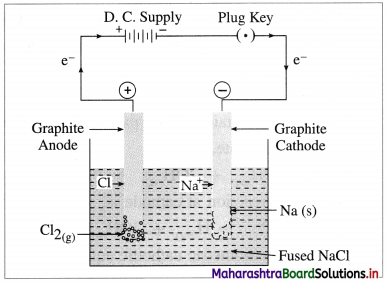
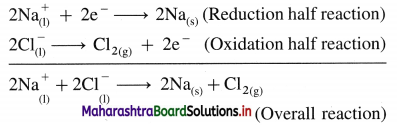
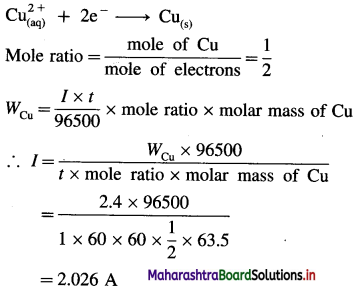
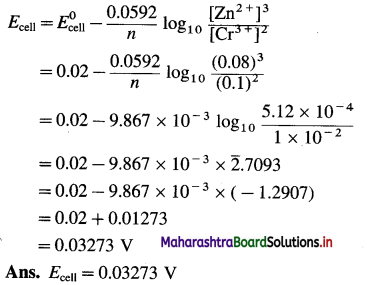
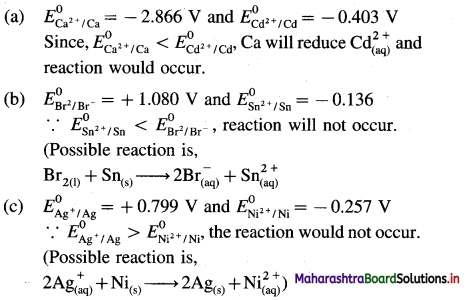
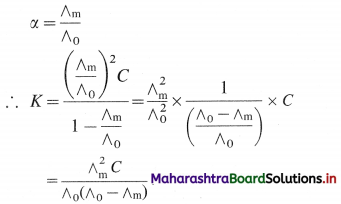
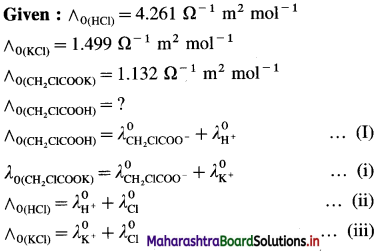
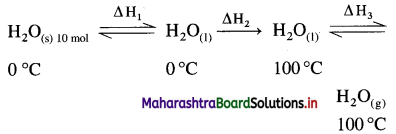
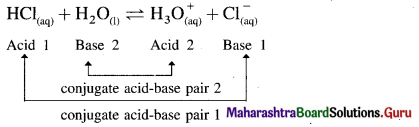
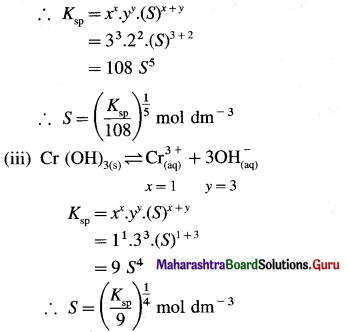
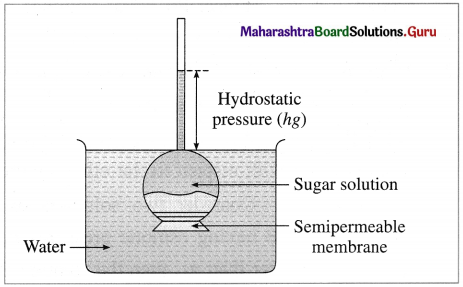
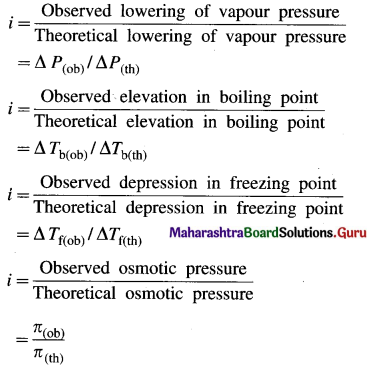
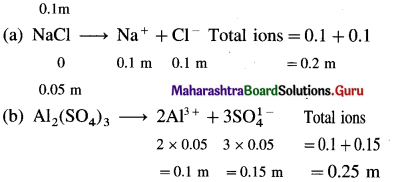
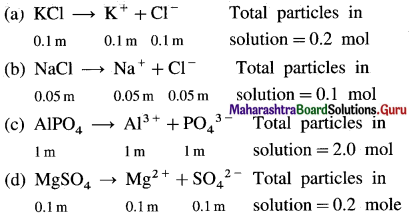
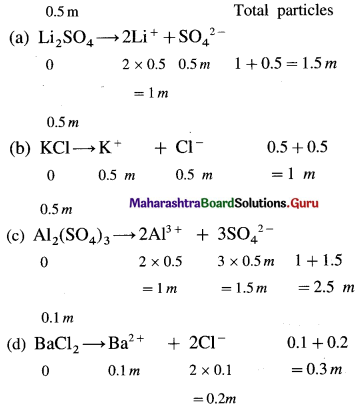
 where [A0] and [A]t are the respective initial and final concentrations of the reactant after time t.
where [A0] and [A]t are the respective initial and final concentrations of the reactant after time t.