Class 9 English Chapter 2.1 Question Answer Maharashtra Board
Balbharti Maharashtra State Board Class 9 English Solutions My English Coursebook Chapter 2.1 Comparisons Notes, Textbook Exercise Important Questions and Answers.
Comparisons Poem 9th Std Question Answer
My English Coursebook Std 9 Digest Chapter 2.1 Comparisons Textbook Questions and Answers
Warming up!
Chit-chat:
- What is your favourite colour?
- What does it remind you of?
- Are you afraid of the dark?
- What does it make you think of?
- If you were asked to design a big garden, what will you include in it?
![]()
Expanding Word Webs:
Question 1.
Form pairs. List the names of as many vehicles as you can. Choose any two of them, but they must be of different types. Then draw a word web for each of them to show their appearance, qualities and the things that these features remind you of. You may use words as well as phrases in the web.
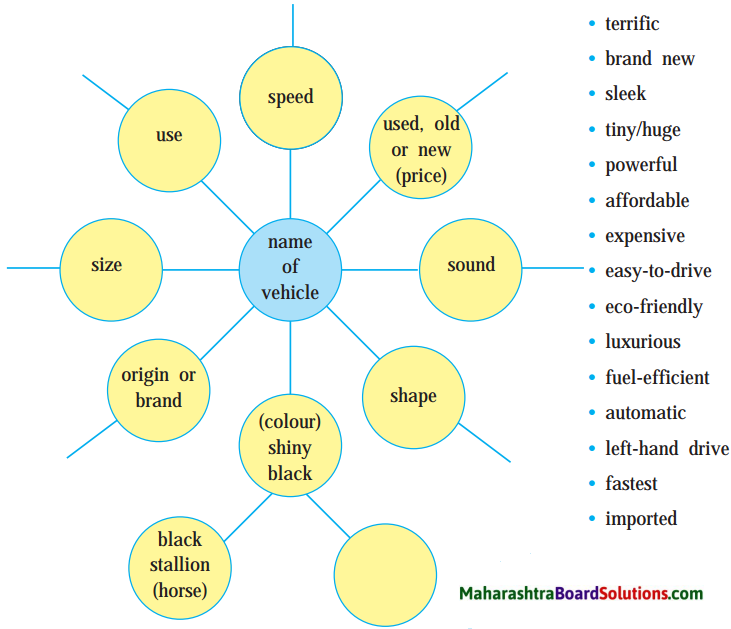
Answer:
Words that can be used in Word-web:
- terrific
- brand-new
- sleek
- tiny/huge
- powerful
- affordable
- expensive
- easy-to-drive
- eco-friendly
- luxurious – arrows.
- fuel-efficient
- automatic
- lefthand drive
- fastest
- imported
Name of the vehicles:
- bicycle
- bullock-cart
- car
- bus
- truck
- motorcycle
- scooter
- boat
- ship
- train
- helicopter
- aeroplane.
Choose any two of above vehicles and write related
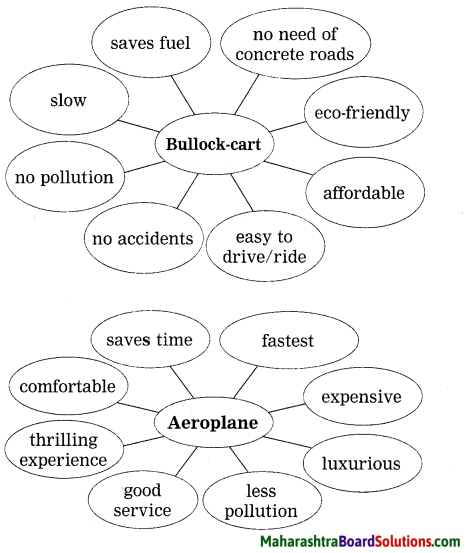
![]()
Question 2.
Note the difference between drive and ride. Use the two words correctly in your own sentences :
Answer:
- Ride – to sit on and be carried by an animal or a two wheeler.
Sentence – I don’t like to ride on a camel. - Drive – to sit in a vehicle and force to go in some directions.
Sentence – My father drives the car with full speed.
Question 3.
Describe your dream vehicle in a few lines :
Answer:
Flying car is my dream vehicle. It will fly in the air. It will fly fast and save our time. There will be no traffic jam problem. No need to construct roads for it. It will save fuel and will be eco-friendly.
![]()
English Workshop:
1. List all the adjectives in the poem. Use the comparative forms of at least 8 of them to write new comparisons. Two of them have been given as examples.
Question 1.
List all the adjectives in the poem. Use the comparative forms of at least 8 of them to write new comparisons. Two of them have been given as examples.
Answer:
- as rapid as spaceship
- as tough as snail
- as dangerous as dinosaur
- as brave as tiger
- as nice as lamb
- as yellow as lemon
- as delicious as jam
- as hot as desert
- as deep as sea
- as fine as house
- as green as pea
- as dark as cave
- as crunchy as toast
- as heavy as road drill
- as fearful as ghost
![]()
2. List all the nouns in the poem. Find a suitable adjective – other than the ones given here – for each of the nouns and write meaningful similes for at least 8 of them.
For example, as yellow as a lemon.
Question 1.
List all the nouns in the poem. Find a suitable adjective – other than the ones given here – for each of the nouns and write meaningful similes for at least 8 of them.
For example, as yellow as a lemon.
Answer:
- as useful as ore
- as lovely as a kitten
- as stable as a rock
- as warm as a mitten
- as narrow as tunnel
- as cute as an elf
- as tricky as a mountain path
- as safe as shelf
![]()
3. Form groups of 6-8. Think of similes using different objects, for example,
Dry as land, Wet as a puddle.
Now try to put together the similes to make a poem with rhyming lines.
Question 1.
Form groups of 6-8. Think of similes using different objects, for example,
Dry as land, Wet as a puddle.
Now try to put together the similes to make a poem with rhyming lines.
4. Write the pairs of rhyming words.
Question 1.
Write the pairs of rhyming words.
Answer:
- kitten – mitten
- moon – noon
- elf – shelf
- shelf – yourself.
- snail – nail
- lamb – jam
- sea – pea
- toast – ghost.
![]()
5. Start a collection of idioms with comparisons. Use the following categories:
Question 1.
Start a collection of idioms with comparisons. Use the following categories:
Answer:
(a) idioms with colours:
- as black as coal
- as white as snow
- as red as blood
- as blue as the sky
- as green as an emerald
- as pink as a rose
- as yellow as gold
- as orange as a pumpkin
- as brown as a coffee bean
(b) Idioms with animals:
- as cunning as a fox
- as brave as a lion
- as hairy as a gorilla
- as hungry as a horse
(c) Idioms with objects:
- as black as coal
- as soft as butter
- as light as cotton
6. Given below are some idiomatic comparisons with ‘like’. Can you guess their meaning? Look them up in a good dictionary. You won’t find them under ‘like’.
Which words will you look up to find these comparisons?
- Like a bull in a china shop (Here, china means delicate articles of porcelain)
- Like a cat on a hot tin roof.
- Like a red rag to a bull.
- Like a cat that stole the cream.
- Like water off a duck’s back.
- Memory like a sieve.
Using your imagination write more comparisons using ‘like’.
![]()
My English Coursebook 9th Class Solutions Chapter 2.1 Comparisons Additional Important Questions and Answers
Read the following extract and do the activities:
Simple Factual Activities:
Question 1.
Read the extract and complete the following by choosing the correct alternative:
(Answers are directly given.)
Answer:
- While comparing a spaceship with a snail, the narrator used speed, as a common feature in both. (speed/weight)
- Square and round are shapes, commonly used to compare, (shapes/objects)
![]()
Question 2.
Match the following:
| ‘A’ | ‘B’ |
| 1. big | (a) toast |
| 2. fierce | (b) desert |
| 3. dry | (c) cave |
| 4. warm | (d) dinosaur |
| (e) tiger |
Answer:
| ‘A’ | ‘B’ |
| 1. big | (d) dinosaur |
| 2. fierce | (e) tiger |
| 3. dry | (b) desert |
| 4. warm | (a) toast |
Question 3.
Answer in 1-2 words:
Answer:
- How is a mountain path? – crooked
- What is the world full of? – opposites
- What is hard and what is soft? – a rock, a mitten
- What is commonly compared in a giant and an elf? – height
![]()
Question 4.
Match the following :
| ‘A’ | ‘B’ |
| 1. dark | (a) elf |
| 2. hard | (b) ox |
| 3. strong | (c) cool |
| 4. short | (d) rock |
| (e) tunnel |
Answer:
| ‘A’ | ‘B’ |
| 1. dark | (e) tunnel |
| 2. hard | (d) rock |
| 3. strong | (b) ox |
| 4. short | (a) elf |
Complex Factual Activity:
Question 1.
Complete the following:
(Answers are directly given.)
Answer:
1. According to the poet a spaceship is fast whereas a snail is slow.
2. The poet says a road drill is noisy and a ghost is quiet.
![]()
Appreciation of Poem:
1. Title: The title of the poem is ‘Comparisons’.
2. Poet: The poem is by an anonymous (= unknown) poet.
3. Theme/Central Idea: The central idea of the poem is given in the last but one line of the poem. The poem celebrates the diversity in the universe. The poem also reminds us that all sorts of people and things are needed to make our world beautiful.
4. Rhyme Scheme: The rhyme Scheme of first six stanzas is ‘abcb’. The last stanza has six lines and its rhyme scheme is ‘abcbdb’.
5. Figure of Speech: Similes.
6. Special Features: The structural quality is a special feature of this poem. The quatrain stanzas and their ‘abcb’ rhyme scheme flows with the force of ballad. Here, in the conclusive two lines, the pace of the poem slows down and poet’s argument sinks deep down in our hearts.
7. Favourite line: The world is full of opposites, so think of some yourself.
8. Why I like the poem: I like this poem because it made me think for the first time the extreme diversity in the world around me. I think the poem helped me in looking at the world around me in a different light.
Vocabulary Focus:
Question 1.
Write adjectives/words used here, to show – shape, size, temperature:
Answer:
- shape – round, square
- size – big, small
- temperature – dry, wet.
![]()
Question 2.
List all the adjectives in the poem. Use the comparative forms to write new comparisons:
Answer:
1. (a) faster than the wind
(b) slower than a tortoise
2. (a) bigger than my brother
(b) smaller than my sister
3. (a) fiercer than a giant
(b) gentler than a butler
4. (a) cooler than ice
(b) warmer than a sweater
5. (a) sourer than tamarind
(b) sweeter than mango
6. (a) noisier than a machine
(b) quieter than a cave.
7. (a) stronger than a tiger
(b) weaker than a rabbit
8. (a) taller than a tree
(b) shorter than plant
9. (a) darker than night
(b) lighter than tubelight
10. (a) harder than a stone
(b) softer than butter
11. (a) crooked than hillroad
(b) straighter than a railway line
My English Coursebook 9th Class Pdf Unit 2