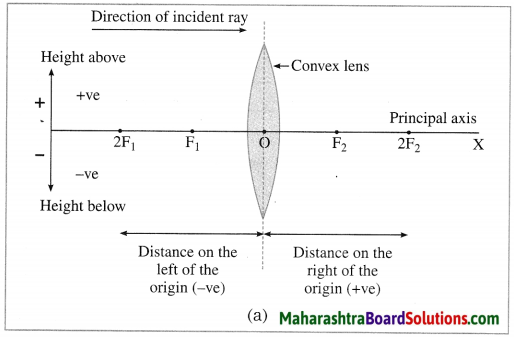
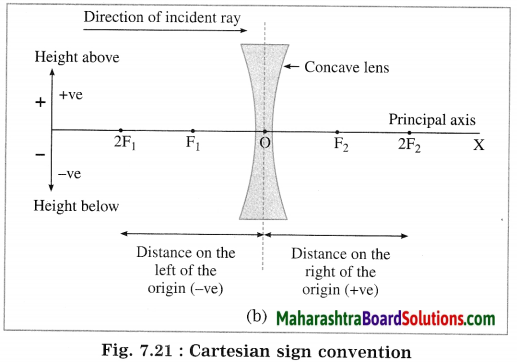
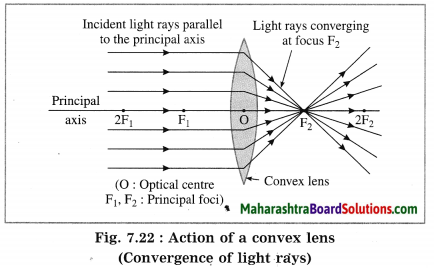
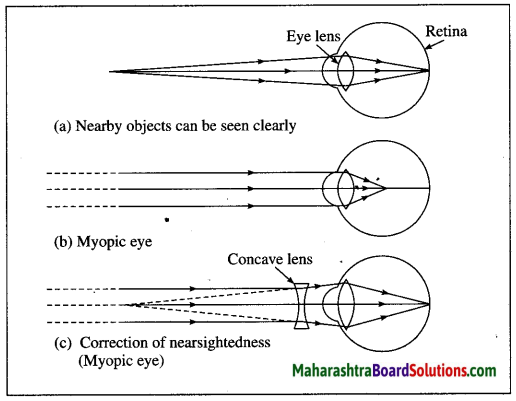
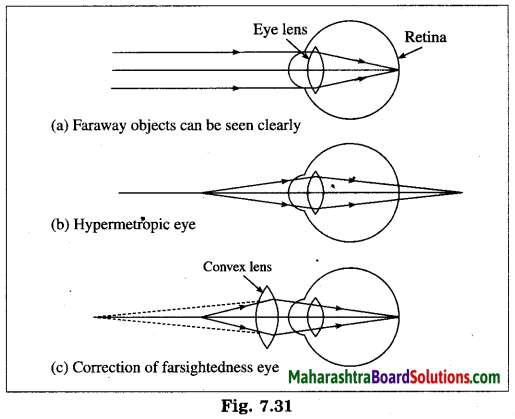
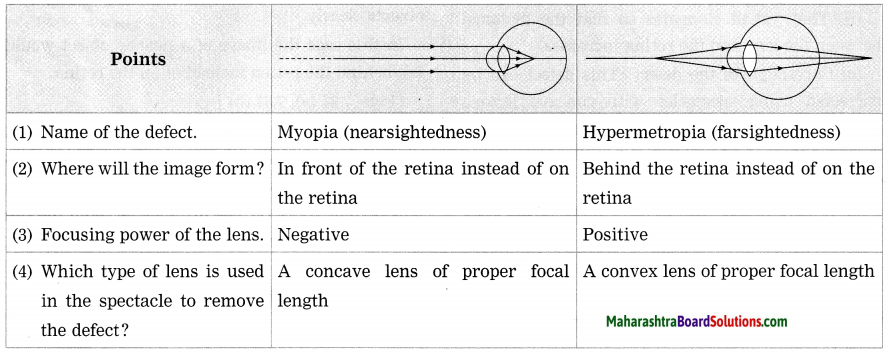
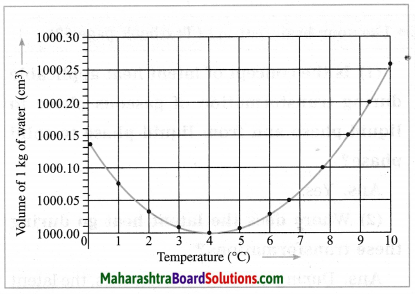
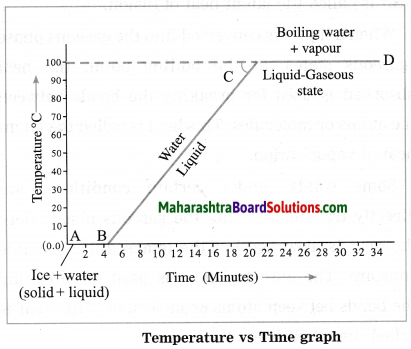
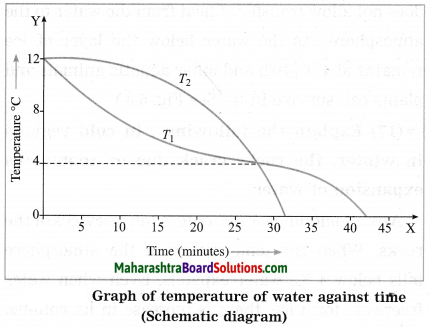
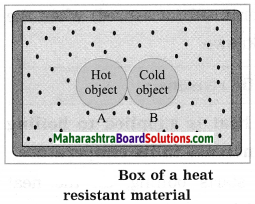
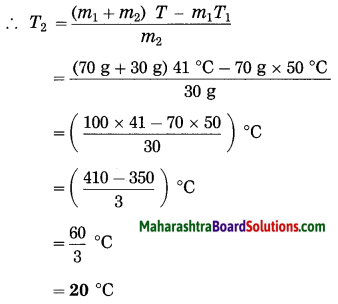
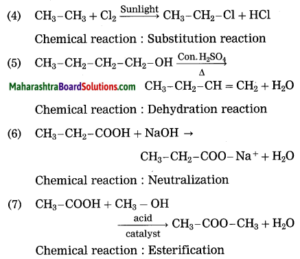
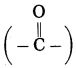
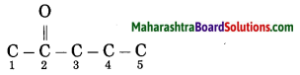
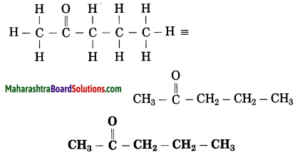
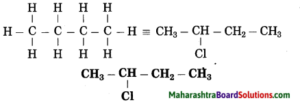
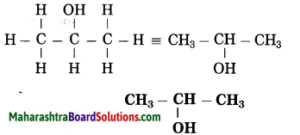
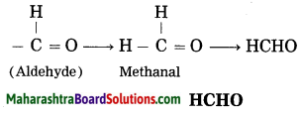
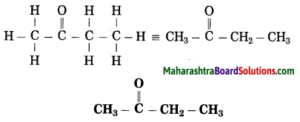
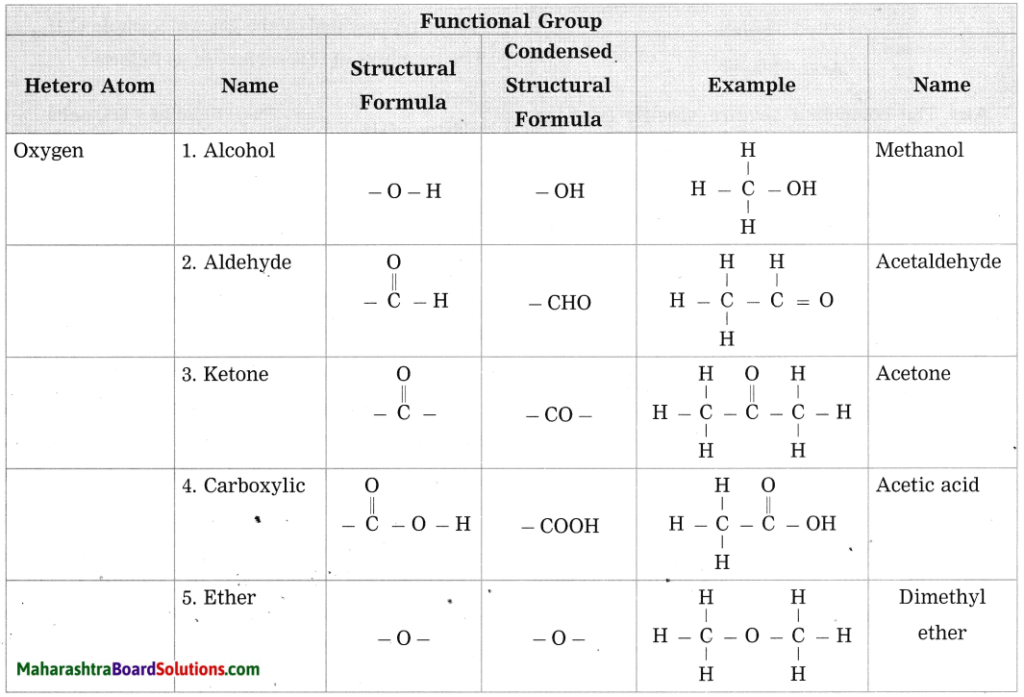
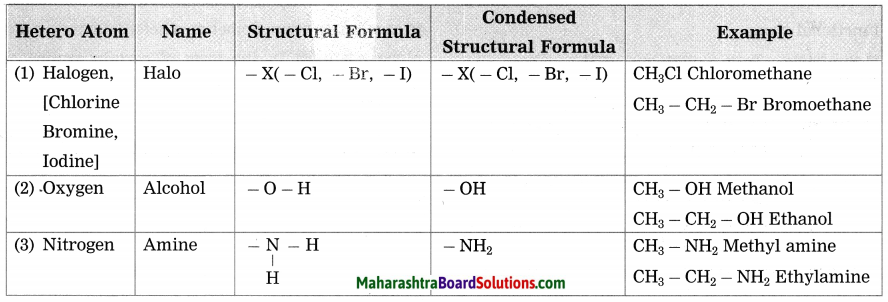
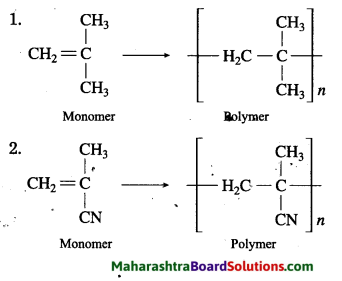
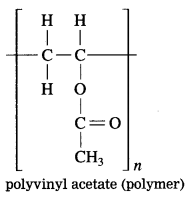
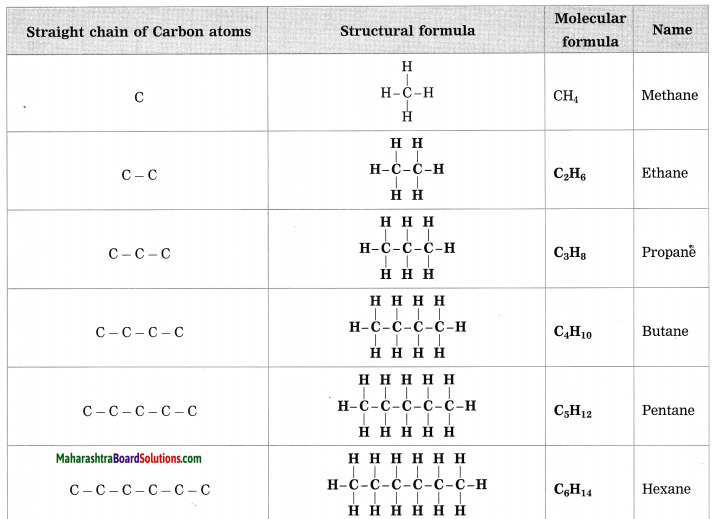
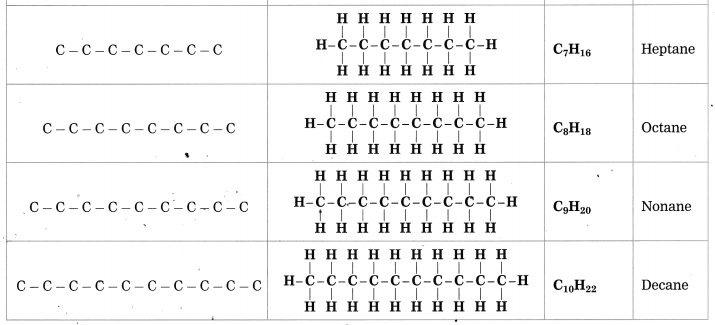
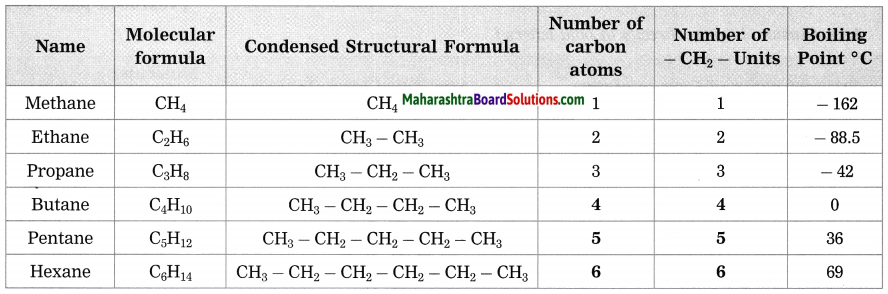
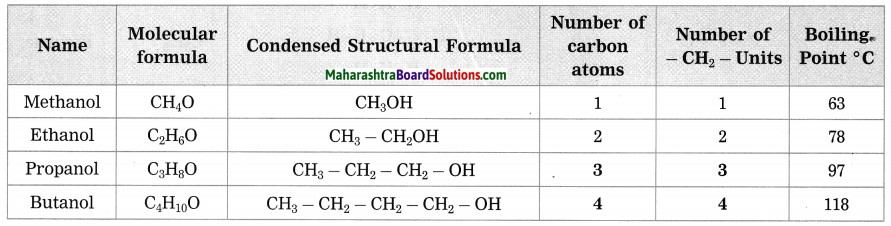
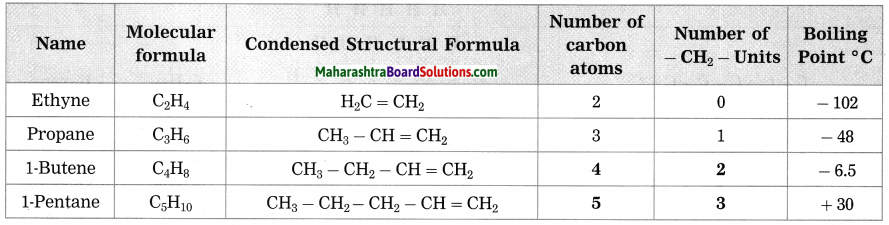
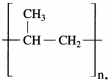
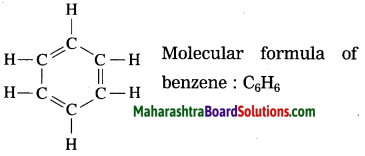
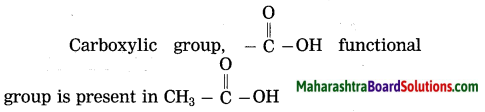
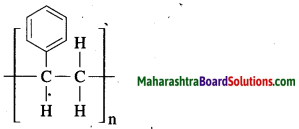
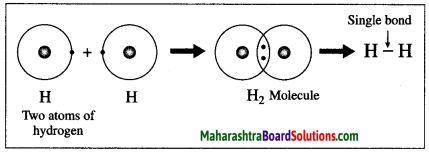
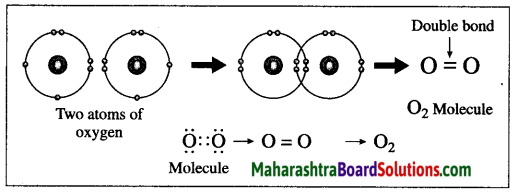
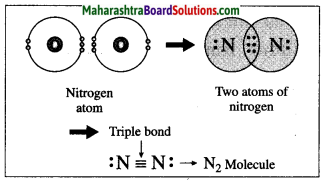
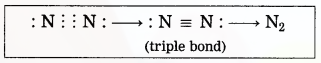
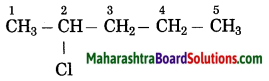
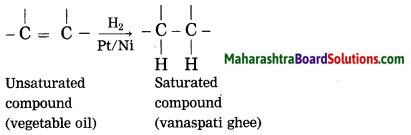
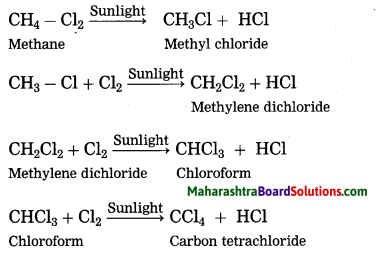
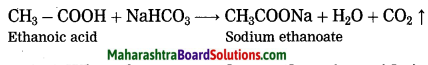
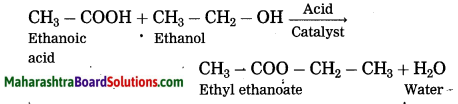
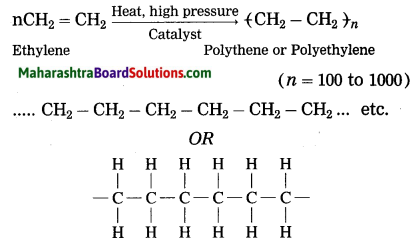
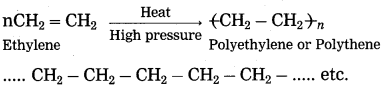
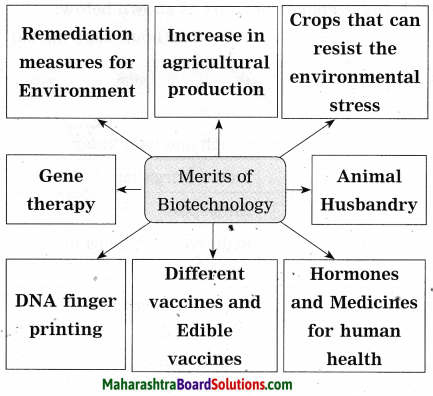
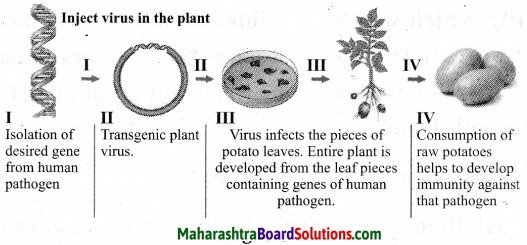
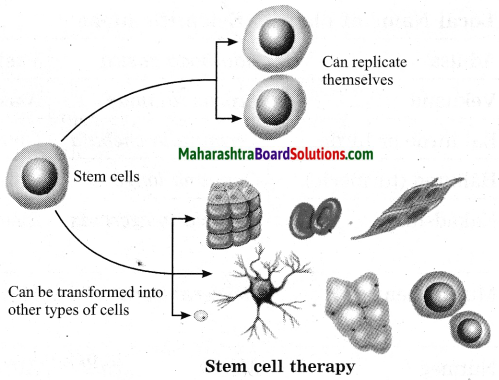
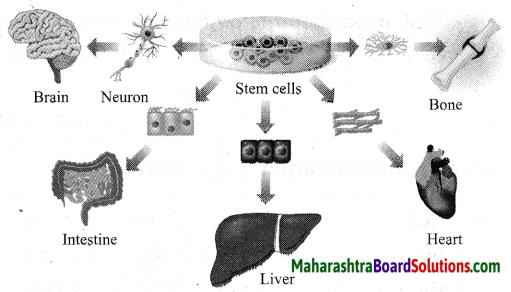
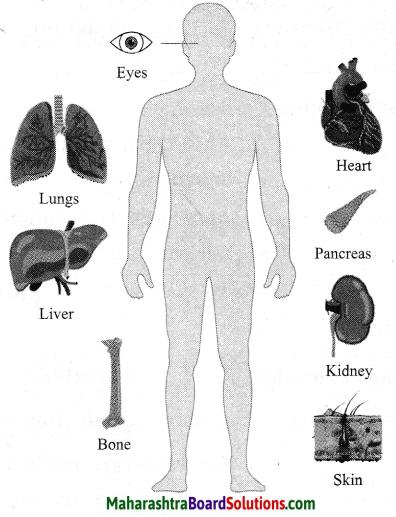
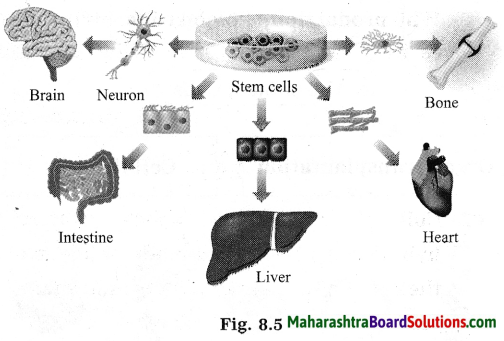
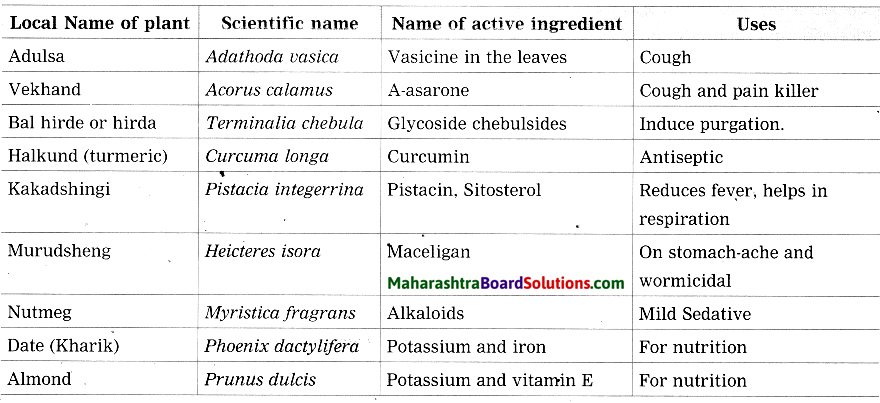
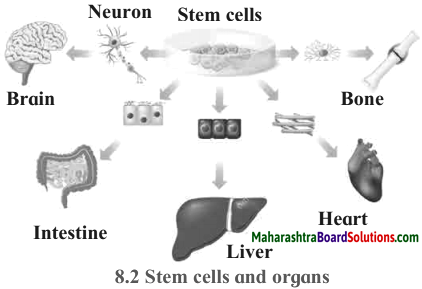
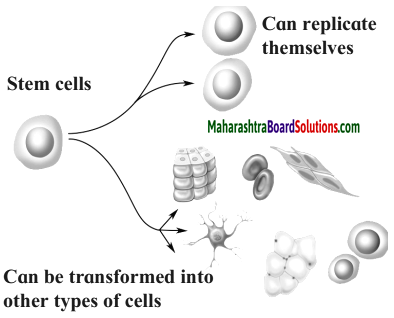
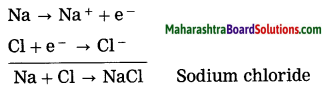
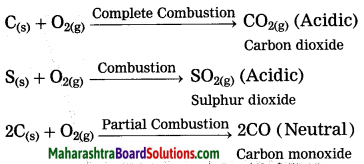
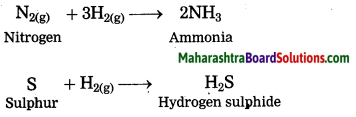
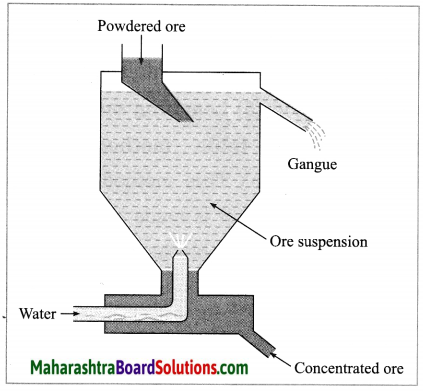
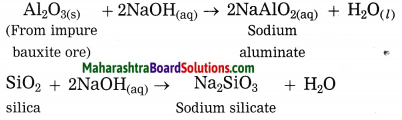
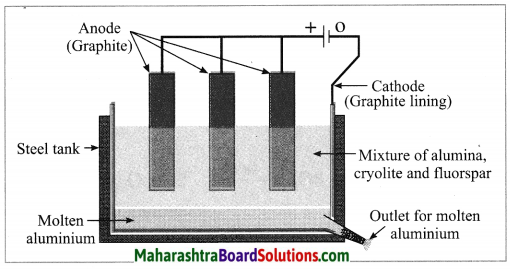
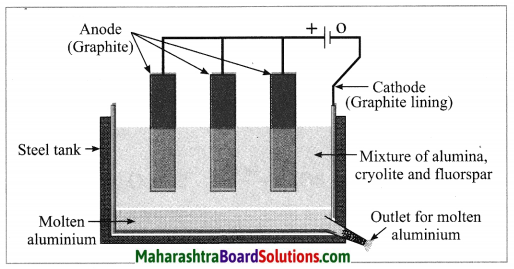
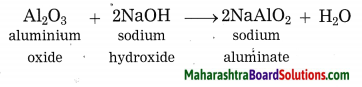
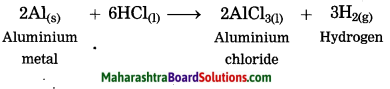
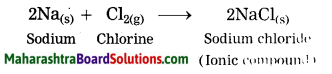
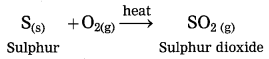
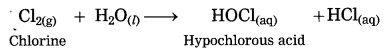
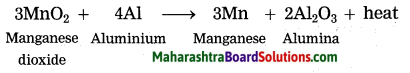
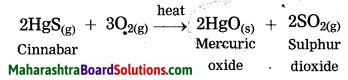
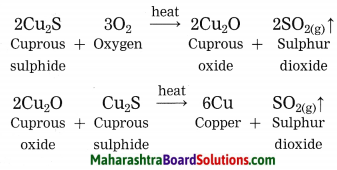
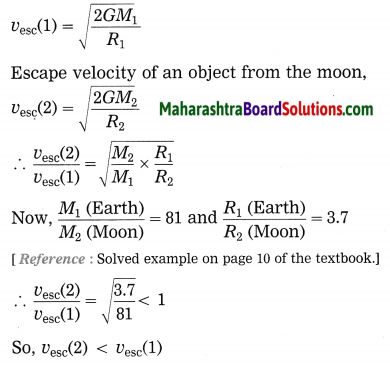
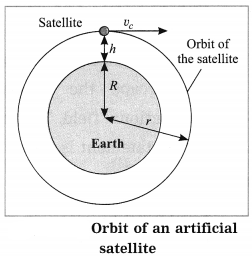
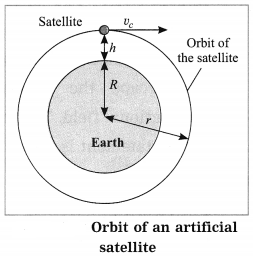
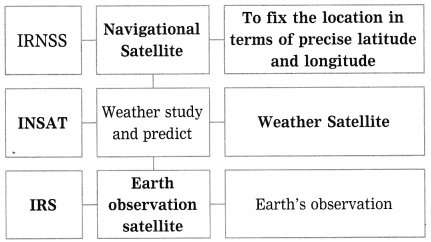
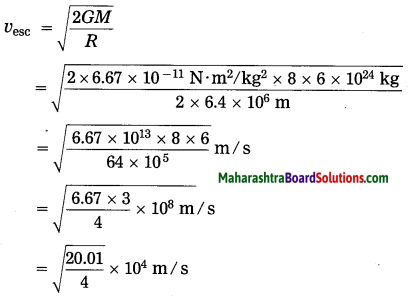
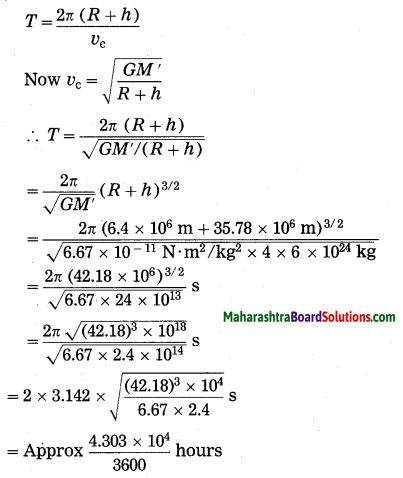
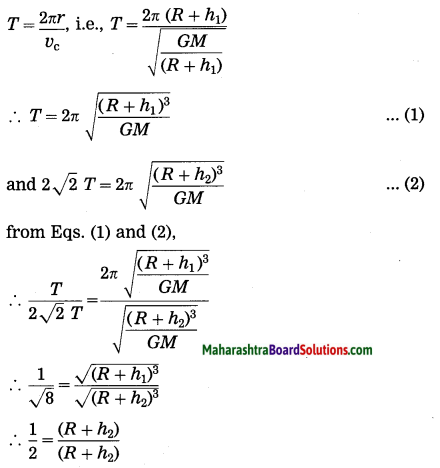
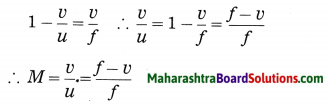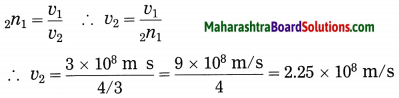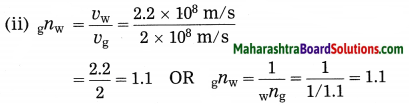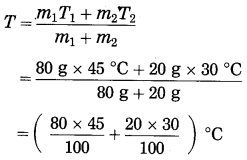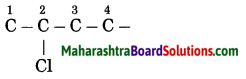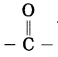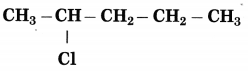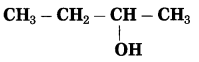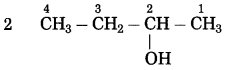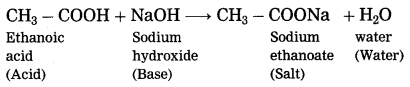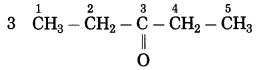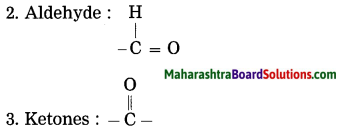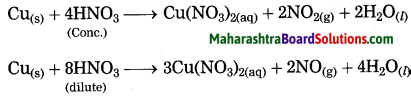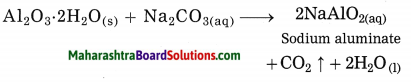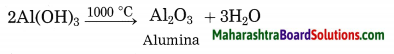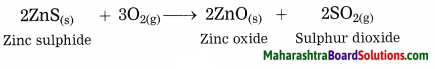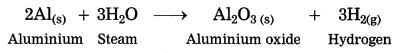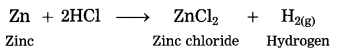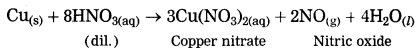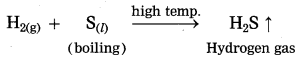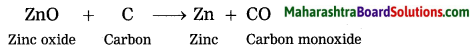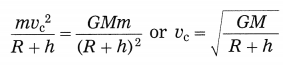Maharashtra State Board Hindi Yuvakbharati 12th Digest Pdf 2021-2022, Hindi Digest Std 12 Pdf Download, Yuvakbharati Hindi 12th Textbook Pdf, 12th Ka Hindi Book Pdf Maharashtra Board, 12th Commerce Hindi Book Guide Question and Answer PDF Free Download.
Maharashtra State Board Yuvakbharati Hindi Digest Std 12 Text Book Solutions Answers Pdf
Hindi Yuvakbharati 12th Digest Pdf | 12th Commerce ka Hindi Book Pdf Maharashtra Board
- Chapter 1 नवनिर्माण
- Chapter 2 निराला भाई
- Chapter 3 सच हम नहीं; सच तुम नहीं
- Chapter 4 आदर्श बदला
- Chapter 5.1 गुरुबानी
- Chapter 5.2 वृंद के दोहे
- Chapter 6 पाप के चार हथियार
- Chapter 7 पेड़ होने का अर्थ
- Chapter 8 सुनो किशोरी
- Chapter 9 चुनिंदा शेर
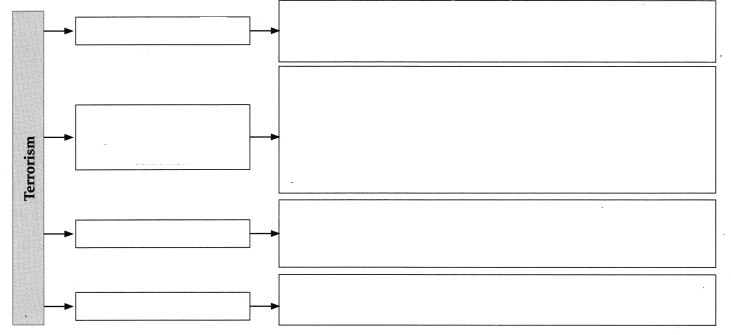
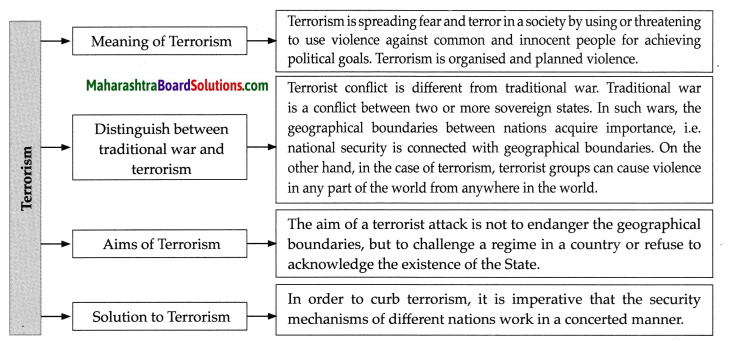
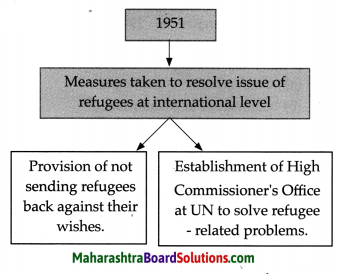
- Chapter 10 ओजोन विघटन का संकट
- Chapter 11 कोखजाया
- Chapter 12 लोकगीत
Hindi Digest Std 12 Pdf Download विशेष अध्ययन हेत
Class 12th Hindi Guide व्यावहारिक हिंदी
- Chapter 14 पल्लवन
- Chapter 15 फीचर लेखन
- Chapter 16 मैं उद्घोषक
- Chapter 17 ब्लॉग लेखन
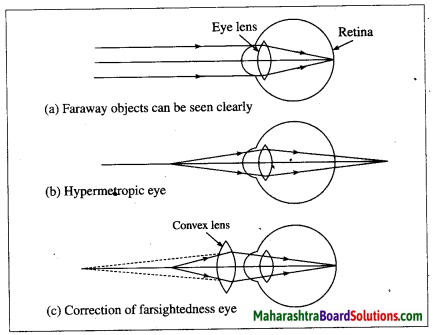
- Chapter 18 प्रकाश उत्पन्न करने वाले जीव
Yuvakbharati Hindi 12th Textbook Pdf परिशिष
- मुहावरे
- भावार्थ : गुरुबानी, वृंद के दोहे
- भावार्थ : सुनु रे सखिया और कजरी
- पारिभाषिक शब्दावली
- अपठित बोध
- व्याकरण