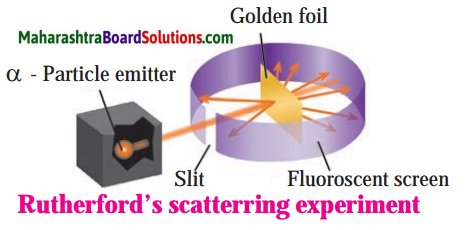
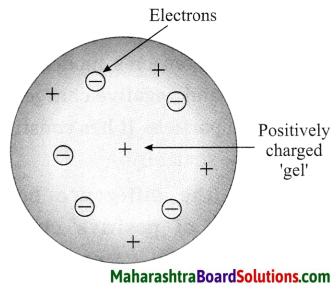
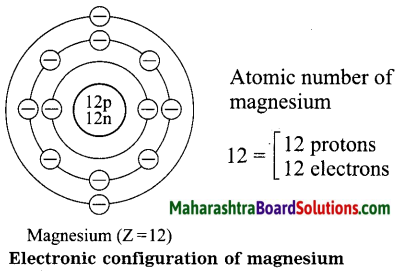
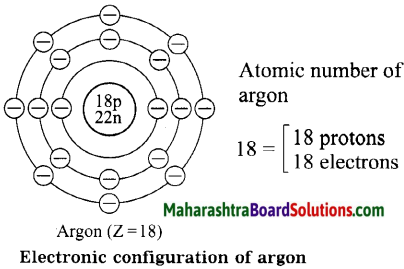
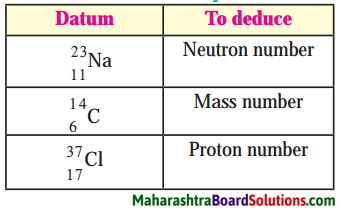
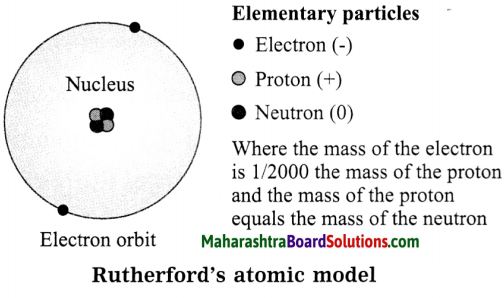
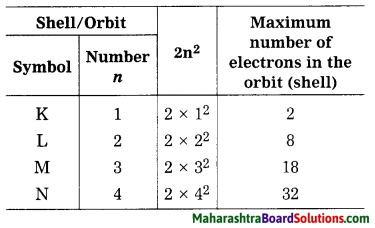
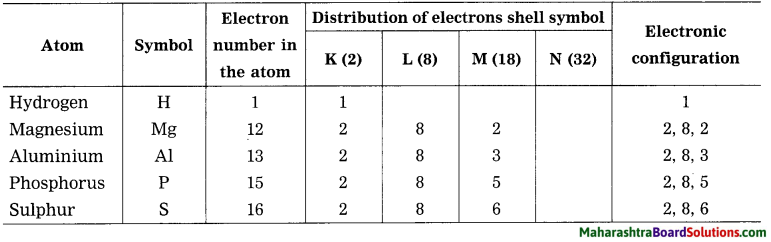
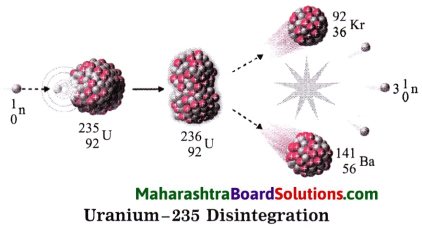
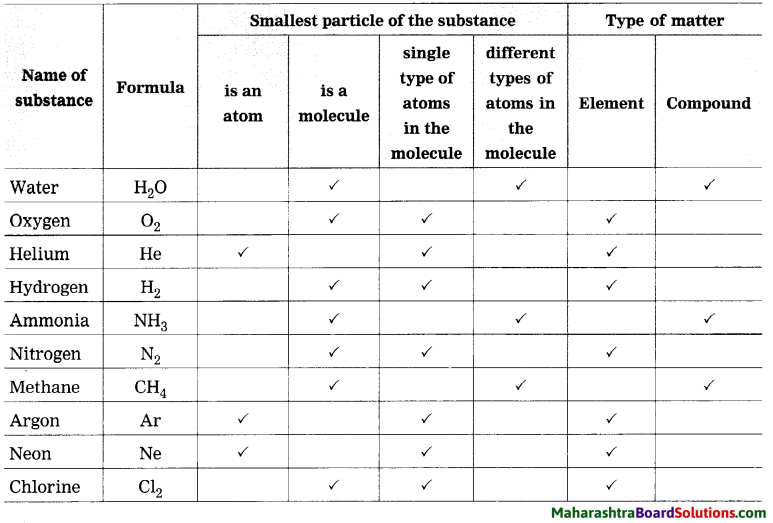
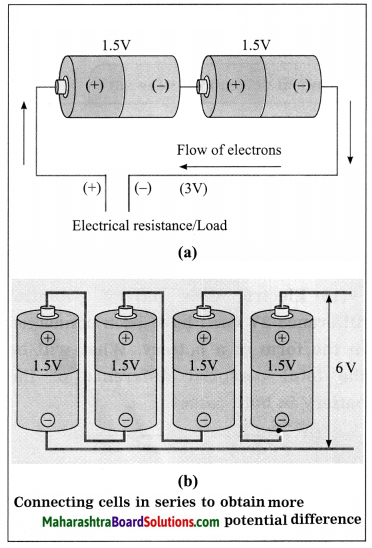
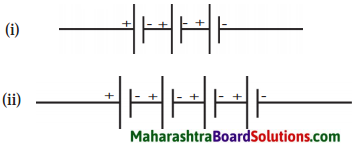
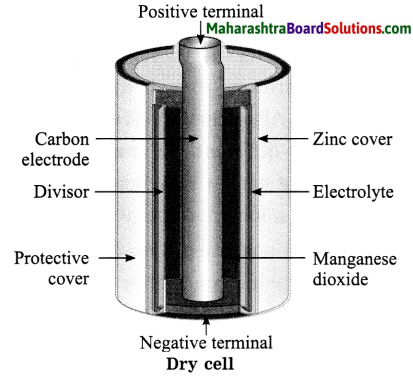
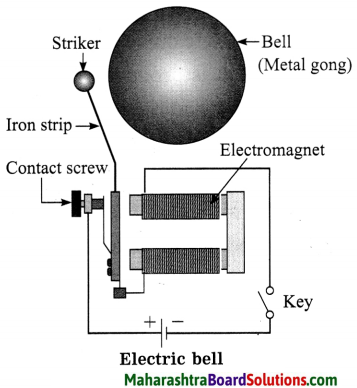
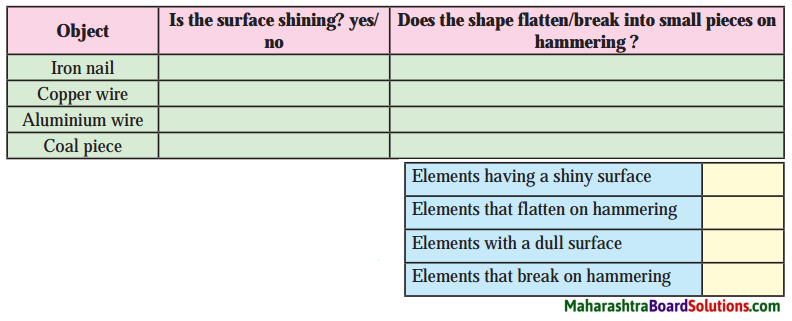
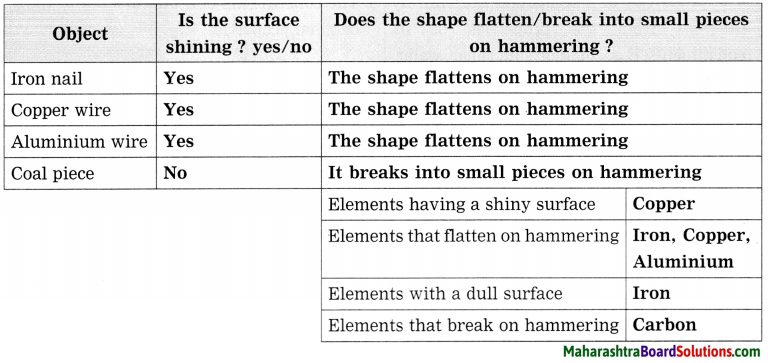
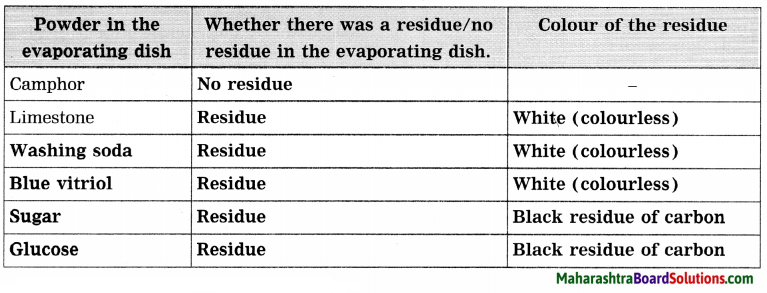
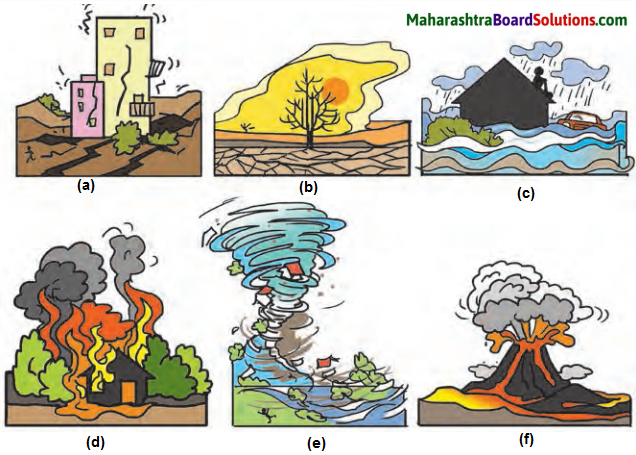
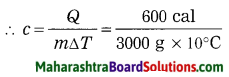
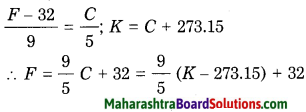Std 8 Science Chapter 9 Disaster Management Question Answer Maharashtra Board
Balbharti Maharashtra State Board Class 8 Science Solutions Chapter 9 Disaster Management Notes, Textbook Exercise Important Questions and Answers.
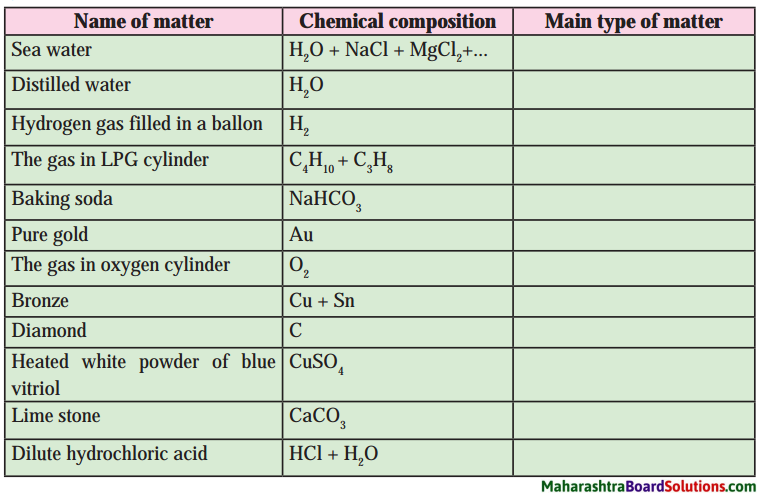
Class 8 Science Chapter 9 Disaster Management Question Answer Maharashtra Board
Class 8 Science Chapter 9 Disaster Management Textbook Questions and Answers
1. Answer the following in your own words.
Question a.
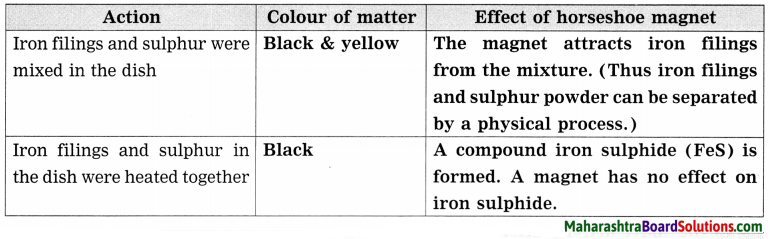
Explain the relation between continuous rains and landslide. Give reasons.
Answer:
Before the actual landslide, many changes occur in the surrounding hilly ground. The hard rocks develop cracks and crevices. These are natural changes but due to man-made activities, the cracks are widened due to erosion. These cracks make the big rocks to break into smaller stones. The cracks widen further due to excessive rainfall. These rocks further get eroded and they fall down along with soil from the slopes. The entire process is speeded up due to rainwater.
![]()
Question b.
Prepare a chart showing ‘Dos’ and ‘don’ts’ at the time of earthquake.
Answer:
At the time of earthquake
| Dos | Don’ts |
| 1. Go in the open grounds. | 1. Don’t wait in the multi-storeyed buildings. Do not use lifts. Use stairs to descend. |
| 2. Keep the electrical appliances and cooking gas closed. | 2. Do not stand near buildings, trees or electric wires and poles. |
| 3. Use battery or torch. | 3. Do not light candles, lantern or match- sticks. |
| 4. Stand silently at one place. Do not panic or get scared. | 4. Do not sit in uncomfortable posture for a long time. |
| 5. Take protection under some hard covering, especially to protect the head and face. Cover the face. | |
| 6. If, in vehicle, find a safe place and stop the vehicle, sit in the vehicle than coming out in open. |
![]()
Question c.
What are the specifications of an earthquake-proof building?
Answer:
The earthquake-proof building is such that even if the earthquake is caused, it should not cause damage to the buildings due to these earth movements. There are some codes of conduct while constructing such buildings. Advanced technology is used for earthquake resistant construction. The foundation of earthquake-proof building is separated from lower land. The walls are of less weight or they are wooden. The house constructed with special light materials are preferred in earthquake-prone regions.
Question d.
Explain the effects of landslide.
Answer:
1. Mostly rivers originate in the hills and mountains. When there is landslide, the rivers naturally get flooded.
2. The paths of the riverine water changes due to landslide.
3. Waterfalls are displaced from their original positions.
4. New and artificial water reservoirs are created.
5. The falling debris, soil and rocks make the trees at the base of hillside uprooted. All the plant life is lost.
6. The constructions done by the villagers on the slope can be totally damaged due to landslide. There is large scale damage to property and life. In few cases like Malin the entire village was buried due to landslide and accompanied rainfall.
7. Transportation is affected as the roads and railway tracks are blocked due to debris.
![]()
Question e.
Is there any relation between dam and earthquake? Explain.
Answer:
There is abundant water stored in the dams. This water column puts additional weight on the ground. Initially there may not be any weight, but later due to construction of dam, suddenly the pressure of this weight is so high that this ground experiences the tension. If such area is already earthquake-prone, then there can be chances of earthquake. According to theory of plate tectonics, there are continuous movements in the earth’s surface. If over such fragile plates, the dams are constructed then the chances of earthquake are enhanced.
2. Give Scientific reasons.
Question a.
It is safer to find shelter under things like a bed, table at the time of earthquake.
Answer:
When earthquake takes place, due to the vibrations in the earth’s surface, there is possibility of the roof and walls of the house to fall. This collapse can cause severe head injury which can be fatal. Thus, one must take shelter below the hard-supporting structures such as bed or table. This precaution can save one’s life.
Question b.
In monsoon, don’t take shelter near hillside.
Answer:
The excessive rainfall can cause landslides. The soil and rocks from the hillside can be pushed down along with the flow of rainwater. This debris slides to the lower heights from the hills. This explains us that taking shelter near the base of the hillside can be disastrous as one can be buried in the debris due to sudden landslide. Therefore, in monsoon, one should not take shelter near hillside.
![]()
Question c.
Don’t use lifts at the time of earthquake.
Answer:
Lifts or elevators run on electricity. The electricity supply can be hindered due to earthquake. There may be chances of fire due to short circuit. We may get trapped in the elevator at such times. The building may also collapse due to earthquake. It is always better to use stairs at the time of such calamities, so that one can safely come out of the building. Therefore, it is said that lifts should not be used at the time of earthquakes.
Question d.
The foundation of earthquake- proof building is separated from lower land.
Answer:
The surface of the earth trembles at the time of earthquake. These tremors cause seismic waves which are responsible for the movements of the earth’s surface. The ground thus shakes or it goes up-down. These shocks and waves formed in the interior of the earth spread on the surface in all directions. This causes collapse of the building and other structures on the land.
To prevent such disasters the foundation of earth-quake proof building is separated from lower land.
3. If a crowd gathers at the place of earthquake, what would be the difficulties in relief work?
Question a.
If a crowd gathers at the place of earthquake, what would be the difficulties in relief work?
Answer:
If people flock in crowd, the rescue work will not be possible. The ambulances and the fire engines will not reach the spot where the help is needed. The personnel from disaster management cells cannot thus act in time. It will be difficult to manage the situation and thus such crowding should never be done.
![]()
4. Make a list of the institutes and organizations who provide help for disaster managment. Collect more information about their work.
Question a.
Make a list of the institutes and organizations who provide help for disaster management. Collect more information about their work.
Answer:
International government organisations working for disaster management are:
a. The United Nations and its organisations:
1. The Food and Agricultural Organisation of the UN (FAO): It gives early warning of impending food crises, and keep track of global food supply problems.
2. The United Nations Development Programme (UNDP): It helps disaster-prone countries with disaster mitigation, prevention and preparedness measures.
3. The World Food Programme (WFP): It is the main supplier of relief food aid.
4. The World Health Organisation (WHO): It gives global public health leadership by setting standards, monitoring health trends, and providing direction on emergency health issues. WHO’s role is to reduce avoidable loss of life and the burden of disease and disability.
b. The International Committee of the Red Cross ( ICRC): It gives physical rehabilitation to people injured by explosive weapons or other types of incident.
c. The International Federation of Red Cross and Red Crescent Societies ( IFRC): It coordinates and gives international help to victims of natural and technological disasters, to refugees and in health emergencies.
International non-governmental agencies working for disaster management are:
a. International Rescue Committee (IRC): It provides lifesaving care and life-changing help to refugees forced to flee from war or disaster.
b. IMA World Health: It, in collaboration with USAID, the World Bank and many other organisations, builds sustainable health care systems.
c. CARE: It is an organisation fighting global poverty. It works for women and puts efforts to improve their basic education, prevent the spread of HIV by providing awareness among them, give them increase access to clean water and sanitation, expand economic opportunity for them. It provides emergency aid to survivors of war and natural disasters, and helps people rebuild their lives.
Indian organisations and institutes working for disaster management are:
a. National Disaster Response Force (NDRF): The multi-disciplinary, multi-skilled, high-tech force of the NDMA are capable of dealing with all types of natural and man-made disasters.
b. National Disaster Management Authority (NDMA): It lay down the policies, plans and guidelines for Disaster Management to ensure timely and effective response to disasters.
c. National Institute of Disaster Management (NIDM): It has been given the responsibilities for human resource development, capacity building, training, research, documentation and policy advocacy in the field of disaster management.
![]()
5. Make a survey of your school according to the plan of disaster management an write the pointwise information.
Question a.
Make a survey of your school according to the plan of disaster managment an write the pointwise information.
Answer:
This an activity based questions. Kindly do it yourself. But make sure to involve the following points in your survey:
- Primary information of the school: It should include information such as name and address of the school and Head Master, total number of school staff and name and phone numbers of school management members.
- School disaster management committee: Get the information of the members involved in disaster management committee.
- Detailed information about school building: Note the number of rooms, classroom, age of the building, types of roofs under this point.
- Information about school ground: It should include information like distance of ground from the main road, types of play grounds.
- Daily routine of the school: It should include information like working time of the school, lunch break time for the school.
- Possible hazards in the school: It should information such as record of past disaster happened in school, current planning for overcoming disasters.
- Disaster management map of the school: It should have information regarding all the buildings of the school, entrances and exit gates, place of probable danger, safer place at the time of disaster.
![]()
6. Are there any possible places of landslide in your area? Collect information from experts.
Question a.
Are there any possible places of landslide in your area? Collect information from experts.
Answer:
This is an activity based question. kindly do it yourself.
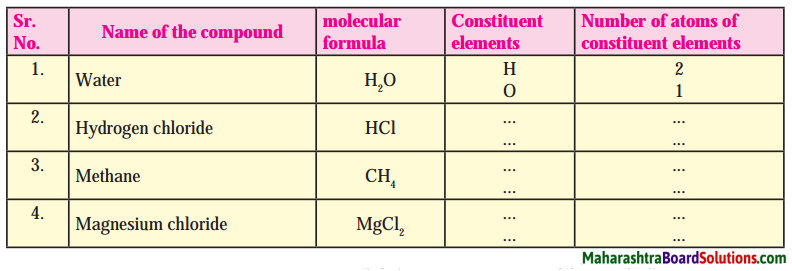
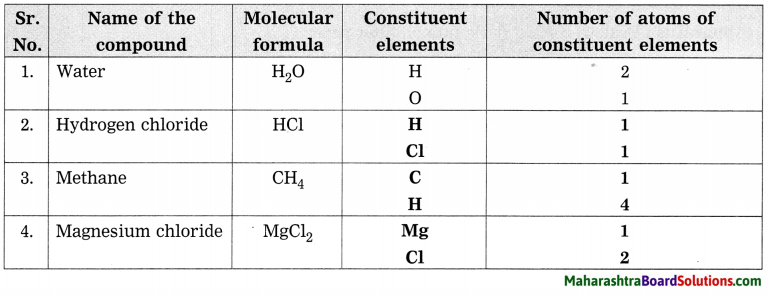
7. With the help of the following pictures, explain your role in disaster management.
Question a.
With the help of following pictures, explain your role in the disaster management.
Answer:

The picture given is not very clear. It does not indicate the condition of disaster. Therefore, there are two alternatives for this answer.
Option 1 : The boy holds a paper on which is written, ‘I am at risk’! The boy is shocked to read this. Someone has given message to him. He must search that person and help him or her. He should therefore take help from some elder or from police force in such matter.
Option 2 : The boy shown in the picture is in danger. So, he is typing in his I – pad, “I am at Risk”. After receiving this message, someone will help him. It depends upon, to whom is he sending this message. If he sends this message to us, we can immediately try to help him. His message “I am at Risk”, shows that he is in danger and has to be saved.
It is not clear about which disaster is shown in the picture.
![]()
Project:
Question 1.
Make a collection of news, photos. and cuttings about landslides and rift collapse.
Question 2.
With the help of Internet, collect information about the latest gadgets and technology to forecast earthquake.
Question 3.
Collect information about NDRF, RPF, CRPF, NCC from Internet.
Question 4.
Discuss- Need of CCTV.
Class 8 Science Chapter 9 Disaster Management Important Questions and Answers
Rewrite the sentences after filling the blanks:
Question 1.
Earthquakes cause ……………. waves leading to movements of the earth’s surface.
Answer:
Earthquakes cause seismic waves leading to movements of the earth’s surface.
Question 2.
The central point of earthquake is the point above the ……………. on the earth’s surface.
Answer:
The central point of earthquake is the point above the epicentre on the earth’s surface.
![]()
Question 3.
The accentuation of earthquake is measured in …………… .
Answer:
The accentuation of earthquake is measured in ‘Richter Scale’.
Question 4.
If there is earthquake at the bottom of ocean, it may create ……………… waves.
Answer:
If there is earthquake at the bottom of ocean, it may create tsunami waves.
Question 5.
…………….. is the best device to put off small fires.
Answer:
Stirrup pump is the best device to put off small fires.
Question 6.
………………. of waterfalls occurs due to landslides.
Answer:
Displacement of waterfalls occurs due to landslides.
State whether the following statements are True or False:
Question 1.
Every year nearly 2,400 to 4,000 earthquakes occur on the earth.
Answer:
False. (Every year nearly 12,400 to 14,000 earthquakes occur on the earth.)
Question 2.
Potassium, sodium and calcium are the metals that react with water at high room temperature.
Answer:
False. (Potassium, sodium and calcium are the metals that react with water at normal room temperature.)
Question 3.
A fire caused due to electrical components is extinguished by fire extinguishers like carbon dioxide are used.
Answer:
True.
![]()
Question 4.
Indiscriminate cutting of the trees results in improvement of soil quality.
Answer:
False. (Indiscriminate cutting of the trees results in soil erosion.)
Question 5.
Landslide results in loss of plant life.
Answer:
True.
Match the column:
Question 1.
| Column ‘A’ | Column ‘B’ |
| 1. Class A fire | a. Electrical components |
| 2. Class B fire | b. Gaseous substances |
| 3. Class C fire | c. Chemical substances |
| 4. Class D fire | d. Liquid substances |
| 5. Class E fire | e. Solid substances |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. Class A fire | e. Solid substances |
| 2. Class B fire | d. Liquid substances |
| 3. Class C fire | b. Gaseous substances |
| 4. Class D fire | c. Chemical substances |
| 5. Class E fire | a. Electrical components |
![]()
Question 2.
| Column ‘A’ | Column ‘B’ |
| 1. Earthquake | a. Formation of artificial water reservoir |
| 2. Tsunami | b. Wildlife lost |
| 3. Forest fire | c. Loss of coastal regions |
| 4. Landslide | d. Change in the level of groundwater-table |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. Earthquake | d. Change in the level of groundwater-table |
| 2. Tsunami | c. Loss of coastal regions |
| 3. Forest fire | b. Wildlife lost |
| 4. Landslide | a. Formation of artificial water reservoir |
Name the following:
Question 1.
Modern equipment to get prior intimation about earthquake:
Answer:
- Laser ranging
- Very long baseline
- Geiger counter
- Creep meter
- Strain meter
- Tide gauge
- Tilt meter
- Volumetric strain gauge.
![]()
Question 2.
Some code of conduct made by Indian Standard Institute for construction of buildings:
Answer:
- IS 456
- IS 1893
- IS 13920
Question 3.
The subcommittees of School Disaster Management.
Answer:
- Fire extinguisher
- Awareness
- Instructions
- Traffic management
- Safety
- Communication committee.
Answer the following questions in one sentence:
Question 1.
Which is the instrument or machine that records the intensity of earthquake?
Answer:
The machine/Instrument which records the earthquakes is called ‘Seismograph’ or ‘Seismometer’.
![]()
Question 2.
Which is the most common and effective solution for extinguishing fire ?
Answer:
Spraying water is the most common and effective solution for extinguishing fire.:
Question 3.
Which type of fires can be extinguished by the method of suppressing?
Answer:
To extinguish the fires caused due to electricity or oil, the method of suppressing the fire can be used.
Question 4.
Which metals react with water at normal room temperature?
Answer:
Combustible metals like potassium, sodium and calcium, react with water at normal room temperature.
Question 5.
Which metals react with water at higher temperatures?
Answer:
Magnesium, aluminum and zinc react with water at high temperatures.
Question 6.
Which institutes have launched a program to forecast the landslides and its effects?
Answer:
The Government of India in collaboration with Indian Mountaineering Institute and International Centre for Integrated Mountain Development has launched a program to forecast the landslides and its effects.
Give scientific reasons:
Question 1.
The electric mains are shut off immediately after the earthquake.
Answer:
There is increased possibility of short circuit after the earthquake. This may result in fire. The earthquake has already – brought the disaster. To prevent further destruction, the electric mains are shut off immediately after the earthquake.
![]()
Write short notes:
Question 1.
Types of fire:
Answer:
There are five types of fire. This division is based on two criteria, viz.
(i) Which substance is being burnt, (ii) What is the method of extinguishing it.
1. Class A Fire: Commonly inflammable solid things such as wood, clothes, coal, papers can be burnt by this type of fire. This fire is extinguished by spraying water over it. This is also called cooling out. Water is effectively used to put off class A fire.
2. Class B Fire: Flammable liquid substances such as petrol, oil, varnish, solvents, cooking oil, paints, etc. catch fire and it is called class B fire. Since these substances are lighter than water, they can be extinguished only by foaming fire extinguishers.
3. Class C Fire: The fire caused due to gaseous substances is called class C fire. Domestic gas (L.P.G.) and acetylene can cause such kind of fire.
4. Class D Fire: Combustible metals catch class D fire. Metals such as potassium, sodium and calcium, can react with water at normal room temperature whereas magnesium, aluminium and zinc react with water at high temperature. When both these groups combine with water, it causes explosion.
5. Class E Fire: When electric components are subjected to fire, they form class C fire. Such fires can be caused by short circuit or due to problems in electric fittings. Such fire is extinguished with the help of carbon dioxide and non-conductive fire extinguishers.
![]()
Question 2.
Disaster relief – planning.
Answer:
Anyone can face disaster at any time. The only way to tackle with such disasters is to keep preparedness to deal with any calamity. Schools, colleges and various offices need to chalk out a detailed planning in case of possible disasters. With this purpose in mind, disaster relief planning is done.
E.g. In the disaster relief planning for school, primary information of the school, the structure of School Disaster Management Committee, detailed information about school building and information about school ground, daily routine of the school such as at what time the school starts and what time does it end, the possible hazards in the school, and disaster management map of the school is included.
Answer the following questions:
Question 1.
What are the different ways to extinguish fire? Write briefly about them.
Answer:
There are three main methods to extinguish the fire. These methods are used to stop the spread of fire and to avoid the financial and other losses.
1. Cooling out by use of water: Water is easily available and can be used for putting off fire instantly. Due to spraying of water, there is cooling effect produced which helps in reducing the loss by fire. Fire can be easily controlled by water.
2. Suppressing the fire by covering it: When there is a fire due to electricity or oil it has to be controlled by covering the fire by sand or soil. When the froth is spread on the fire, there is no contact between air and fire. This puts off the fire and stops the spread of fire caused due to oil.
3. Keep away Flammable Substances: Care is taken to keep away all flammable substances from the fire. Wooden articles and inflammable substances are kept in such as way the fire will not engulf it. Stirrup pumps are used to put off small fires by which the water is spread in all directions around the fire.
![]()
Question 2
What are the safety measures and precautions to stop the fire?
Answer:
- Switching off the regulator of cooking gas cylinder when not in use. Put off the connections of all electrical appliances when not in use.
- If there is fire, call others immediately for help. Take help of others by calling them. Also help others who are in need to save their lives from fire.
- Get help from fire brigade by calling phone number 101.
- Know details about working of the fire extinguishing apparatus.
- Give first aid to the victim of fire. Seek immediate medical help,
Question 3.
What are the causes of landslide?
Answer:
- Various types of natural disasters like earthquakes, tsunami, heavy rains, storms, floods can result into landslide.
- The indiscriminate cutting down of the trees can result into soil erosion. Soil erosion in turn results into landslide.
- The construction of roads, bridges, railway tracks, etc. on the mountain slopes result into lot of digging. Such activities make soil cover loose. From the slopes the soil and rocks then can slide easily. This results into landslides.
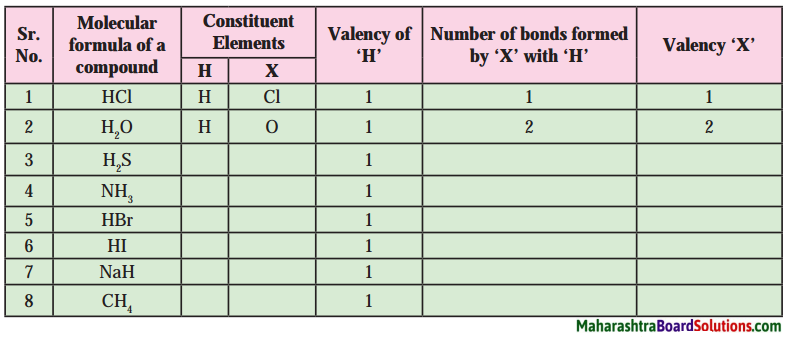
Draw a well labelled diagrams:
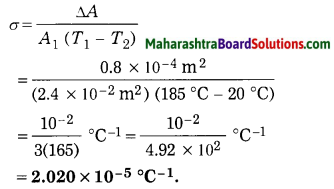
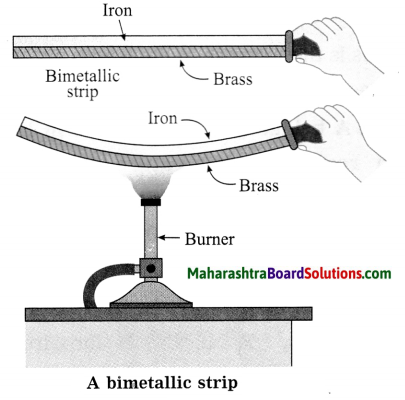
Question 1
Seismometer/Secismograph.
Answer:
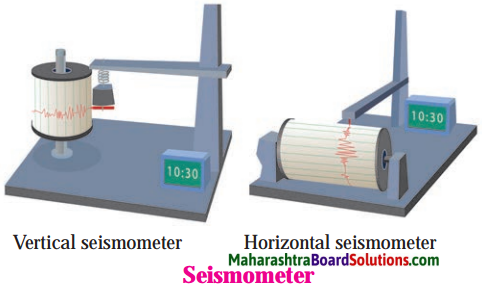
Question 2.
Focal point and epicenter or earthquake.
Answer:
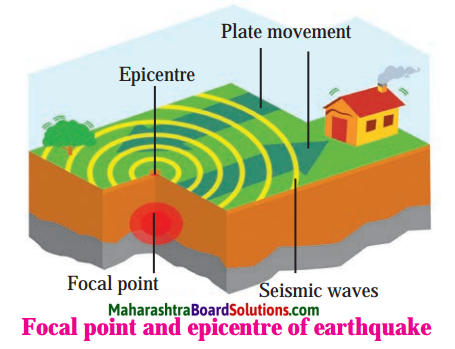
Open-Ended Questions:
Question 1.
Have you at any time faced with any disaster? What were your experiences during such condition? How did you get rescued?
Answer:
Students should write their own experiences about such incidents if any.
![]()
Question 2.
In Maharashtra where do the traffic jams occur due to landslides? Make a list of such places. Why does the landslide occur at those places only? Discuss in the classroom and suggest preventive measures.
Answer:
In Maharashtra, the landslides are very common in the hilly regions. The traffic is suspended often during rainy season when such calamity strikes. Malshej, Khandala, Kasara are some of the Ghats which are very prone to land-slide. On the tracks of Konkan railway, the landslides are very common during heavy downpour.
The regions where more landslides occur have many constructions and large scale deforestation. The traffic of Konkan railway comes to standstill after such landslide. Moreover, during heavy monsoon days, the speed of the running trains is kept very slow. The soil structure is also responsible for such landslides.
Preventive measures: There should be wire meshing done across the sides of the roads and railway tracks so that the collapsed rocks and other debris does not obstruct the traffic. Tree plantation should be carried out to prevent loosening of the soil and rock. It will also help in preventing the soil erosion.
Balbharati Maharashtra State Board 8th Std Science Textbook Solutions
- Living World and Classification of Microbes Class 8 Science Textbook Solutions
- Health and Diseases Class 8 Science Textbook Solutions
- Force and Pressure Class 8 Science Textbook Solutions
- Current Electricity and Magnetism Class 8 Science Textbook Solutions
- Inside the Atom Class 8 Science Textbook Solutions
- Composition of Matter Class 8 Science Textbook Solutions
- Metals and Nonmetals Class 8 Science Textbook Solutions
- Pollution Class 8 Science Textbook Solutions
- Disaster Management Class 8 Science Textbook Solutions
- Cell and Cell Organelles Class 8 Science Textbook Solutions