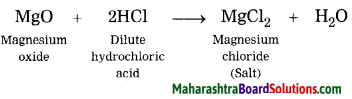
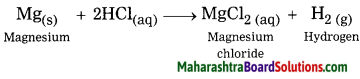
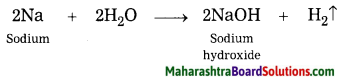
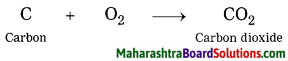
Inheritance and Variation Class 12 Exercise Question Answers Solutions Maharashtra Board
Balbharti Maharashtra State Board 12th Biology Textbook Solutions Chapter 3 Inheritance and Variation Textbook Exercise Questions and Answers.
Class 12 Biology Chapter 3 Exercise Solutions Maharashtra Board
Biology Class 12 Chapter 3 Exercise Solutions
1. Multiple Choice Questions
Question 1.
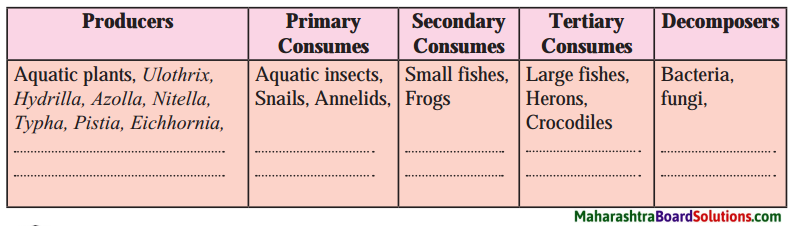
Phenotypic ratio of incomplete dominance in Mirabilis jalapa.
(a) 2 : 1 : 1
(b) 1 : 2 : 1
(c) 3 : 1
(d) 2 : 2
Answer:
(b) 1 : 2 : 1
Question 2.
In dihybrid cross, F2 generation offspring show four different phenotypes while the genotypes are ……………….
(a) six
(b) nine
(c) eight
(d) sixteen
Answer:
(b) nine
![]()
Question 3.
A cross between an individual with unknown genotype for a trait with recessive plant for that trait is ……………….
(a) back cross
(b) reciprocal cross
(C) monohybrid cross
(d) test cross
Answer:
(d) test cross
Question 4.
When phenotypic and genotypic ratios are the same, then it is an example of ……………….
(a) incomplete dominance
(b) complete dominance
(c) multiple alleles
(d) cytoplasmic inheritance
Answer:
(a) incomplete dominance
Question 5.
If the centromere is situated near the end of the chromosome, the chromosome is called ……………….
(a) Metacentric
(b) Acrocentric
(c) Sub-Metacentric
(d) Telocentric
Answer:
(d) Telocentric
Question 6.
Chromosomal theory of inheritance was proposed by ……………….
(a) Sutton and Boveri
(b) Watson and Crick
(c) Miller and Urey
(d) Oparin and Halden
Answer:
(a) Sutton and Boveri
Question 7.
If the genes are located in a chromosome as p-q-r-s-t, which of the following gene pairs will have least probability of being inherited together ?
(a) p and q
(b) r and s
(c) s and t
(d) p and s
Answer:
(d) p and s
Question 8.
Find the mismatched pair:
(a) Down’s syndrome = 44 + XY
(b) Turner’s syndrome = 44 + XO
(c) Klinefelter’s syndrome = 44 + XXY
(d) Super female = 44 + XXX
Answer:
(a) Down’s syndrome = 44 + XY
Question 9.
A colourblind man marries a woman, who is homozygous for normal colour vision, the probability of their son being colour blind is ……………….
(a) 0%
(b) 25%
(c) 50%
(d) 100%
Answer:
(a) 0%
2. Very Short Answer Questions
Question 1.
Explain the statements
a. Test cross is back cross but back cross is not necessarily a test cross.
b. Law of dominance is not universal.
Answer:
a. (1) Test cross is the cross between F1 hybrid and its homozygous recessive parent.
(2) Back cross is the cross of offspring with any one of the parents, either dominant or recessive.
(3) Therefore, test cross can be a back cross – but back cross cannot be a test cross.
b. (1) There are many traits in many organisms which show dominance. For example, widow’s peak in human beings is a dominant trait. Yellow seed colour and round seed shape are dominant traits in pea plant.
(2) However, there are characters which are either co-dominant, such as genes for human blood group A and B or incompletely dominant as in flower colour of Mirabilis jalapa.
(3) Therefore the law of dominance is not universally applicable.
Question 2.
Define the following terms:
a. Dihybrid cross
b. Homozygous
c. Heterozygous
d. Test cross
Answer:
a. A cross between parents differing in two heritable traits is called dihybrid cross.
b. An individual possessing identical alleles for a particular trait is called homozygous or pure for that trait. E.g. TT for tallness and tt for dwarfness.
c. An individual possessing contrasting allele for a particular trait is called heterozygous. E.g. Tt showing tallness.
d. The cross of F1 progeny with homozygous recessive parent is called a test cross.
![]()
Question 3.
What are allosomes?
Answer:
Allosomes are the chromosomes which decide the sex of an organism.
Question 4.
What is crossing over?
Answer:
Crossing over is the process of forming new recombinations by interchanging and exchanging non-sister chromatid arms of the homologous chromosomes.
Question 5.
Give one example of autosomal recessive disorder.
Answer:
Thalassemia is an example of autosomal recessive disorder.
Question 6.
What are X-linked genes?
Answer:
Genes located on the non-homologous region of X chromosome are called X-linked genes.
Question 7.
What are holandric traits?
Answer:
Genes located on the non-homologous region of Y chromosome are called Y-linked genes. The traits due to such genes are called holandric traits which are seen only in male sex.
Question 8.
Give an example of chromosomal disorder caused due to non-disjunction of autosomes.
Answer:
Down’s syndrome is an example of chromosomal disorder caused due to non-disjunction of autosomes.
Question 9.
Give one example of complete sex linkage.
Answer:
Sex linkage can be complete X linkage and complete Y linkage. X linkage is haemophilia and Y linkage is hypertrichosis.
3. Short Answer Questions
Question 1.
Enlist seven traits of pea plant selected / studied by Mendel.
Answer:
Seven traits in pea selected by Mendel:
- Tall habit versus dwarf habit (Height of the plant).
- Purple flowers versus white flowers. (Colour of flowers)
- Yellow seeds versus green seeds. (Colour of seeds)
- Round seeds versus wrinkled seeds. (Shape of seeds)
- Green pods versus yellow pods. (Colour of pods)
- Inflated pods versus constricted pods. (Shape of pods)
- Axial flower versus terminal flower. (Position of a flower)
Question 2.
Why law of segregation is also called the law of purity of gametes?
Answer:
(1) Mendel’s law of segregation is also called Law of purity of gametes because, during formation of gametes, the alleles separate/ segregate from each other and only one allele enters a gamete.
(2) The separation of one allele does not affect other. Since single allele enters a gamete means gametes will be pure for a trait.
E.g. The contrasting characters such as tall (T) and dwarf (t) present in F1 hybrid (Tt) segregate during the formation of gametes.
(3) Owing to this, two types of gametes i.e. T and t are formed which are pure for the characters which they carry.
(4) Thus for example:
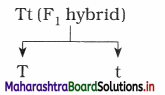
Question 3.
Pleiotropy.
Answer:
- When a single gene controls two or more different traits, it is called a pleiotropic gene and the phenomenon is known as pleiotropy or pleiotropism.
- The pleiotropic ratio is always 1 : 2 instead . of normal 3 : 1.
- Sickle-cell anaemia is caused by the gene HbS. The healthy or normal gene which is dominant is HbA. The heterozygotes or carriers i.e., HbA/Hbs show anaemia as there is deficiency of haemoglobin due to sickling of RBCs. Abnormally low concentration of oxygen can cause sickling of RBCs.
- The homozygotes possessing the recessive gene HbS die because of fatal anaemia because the gene for sickle-cell anaemia is lethal in homozygous condition and causes sickle-cell trait in heterozygous carrier.
- Thus a single gene produces two different expressions.
- When two carriers are married they will produce normal carriers and Sickle-cell anaemic children in the ratio of 1 : 2 : 1. Out of these three children sickle-cell anaemic child will die leaving the ratio 1 : 2 instead of 3 : 1.
![]()
Question 4.
What are the reasons of Mendel’s success?
Answer:
Reasons for Mendel’s success:
- Mendel planned his experiments carefully and these experiments consisted of large sample.
- He always recorded the results of number of plants of each type and their ratios.
- The contrasting characters that he chose were easily recognizable.
- The seven pairs of contrasting characters that he selected were under control of a single factor each. They were present on separate chromosomes and were transmitted from one generation to the next.
- Mendel studied and introduced concept of dominance and recessiveness.
Question 5.
“Father is responsible for determination of sex of child and not the mother”. Justify.
Answer:
- Human made is heterogame tic, i.e. he produces two different types of sperms. One is bearing X chromosome along with 22 autosomes and the other is Y bearing sperm with 22 autosomes.
- Mother, on the other hand, is homogametic, producing all similar types of ova, i.e 22 + X chromosomal combination.
- If 22+X bearing sperm fertilise an egg, female child is formed while if Y bearing sperm fertilizes an egg, male child is formed.
- Thus the sex of the child is dependent upon type of sperm that father gives, therefore, it is said that father is responsible for determination of sex of a child and not the mother.
Question 6.
What is linkage? How many linkage groups do occur in human being and maize?
Answer:
- Linkage is defined as the tendency of the genes to be inherited together because they are present in the same chromosome. Linkage group is group of genes situated on a chromosome.
- Humans have 23 linkage groups because they have 23 pairs of chromosomes.
- Maize plant has 10 linkage groups because they have 10 pairs of chromosomes.
Question 7.
PKU.
Answer:
- PKU means phenylketonuria which is an autosomal recessive inborn error.
- In this disorder the metabolism of phenylalanine does not occur due to deficiency of phenylalanine hydroxylase (PAH) enzyme.
- This enzyme is necessary to metabolize the amino acid phenylalanine to the amino acid tyrosine.
- When PAH activity is reduced, phenylalanine accumulates in blood and cerebrospinal fluid and is converted into phenylpyruvate or phenyl-ketone which is a toxic compound. This may cause mental retardation. Excess phenylalanine is excreted in urine, hence this disease is called phenylketonuria.
- PKU is caused by mutations in the PAH gene on chromosome no. 12.
- Untreated PKU causes abnormal phenotype which includes growth failure, poor skin pigmentation, microcephaly, seizures, global developmental delay and severe intellectual impairment. However, at birth if an infant is checked for PKU, the further abnormalities can be avoided.
Question 8.
Compare X-chromosome and Y-chromosome.
Answer:
| X-chromosome | Y-chromosome |
| 1. X-chromosome is straight, rod like and longer 1. than Y chromosome. It is metacentric. | 1. Y-chromosome is shorter chromosome which is acrocentric. |
| 2. X-chromosome has large amount of euchromatin and small amount of heterochromatin. | 2. Y-chromosome has small amount of euchromatin and large amount of heterochromatin. |
| 3. X-chromosome has large amount of DNA, hence it is genetically active due to more genes. | 3. Y-chromosome has less amount of DNA, hence it is genetically less active or inert due to lesser genes. |
| 4. Non-homologous region of X-chromosome is longer and contains more genes. | 4. Non-homologous region of Y-chromosome is shorter and contains lesser genes. |
| 5. Contains X-linked genes on non-homologous region. | 5. Contains Y-linked genes on non-homologous region. |
| 6. X-chromosome is present in men as well as women. | 6. Y-chromosome is present only in men. |
Question 9.
Explain the chromosomal theory of inheritance.
Answer:
Chromosomal theory of inheritance was put forth by Sutton and Boveri after studying paraillel behaviour of genes and chromosomes during meiotic division. This theory states following points:
- Chromosomal theory identifies chromosomes as the carrier of genetic material.
- All the hereditary characters are transmitted by gametes. Nucleus of gametes, i.e. sperms and ova of the parents contain chromosomes which transmit the heredity to offspring.
- Chromosomes are found in pairs in somatic or diploid cells.
- During gamete formation, homologous chromosomes pair and segregate independently at meiosis. The diploid condition is converted into haploid condition. Thus each gamete contains only one chromosome of a pair.
- During fertilization, the union of sperm and egg restores the diploid number of chromosomes.
Question 10.
Observe the given pedigree chart and answer the following questions
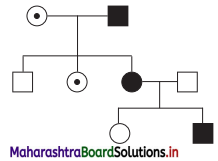
(a) Identify whether the trait is sex-linked or autosomal.
(b) Give an example of a trait in human beings which shows such a pattern of inheritance.
Answer:
Pedigree given above shows:
- First Generation : Carrier woman marrying a sufferer man. Their three children are in following birth order.
- Second generation : First son is normal, second daughter is carrier and third daughter is sufferer.
- Third generation : The sufferer daughter marries a normal man. Her children are normal daughter and sufferer son.
(a) The above pedigree show sex-linked (X-linked) trait. Since criss-cross inheritance is seen in the trait, it must be sex-linked inheritance.
(b) Such trait and its inheritance can be seen in colour blindness.
4. Match the Columns
rewrite the matching pairs.
| Column I | Column II |
| (1) 21 trisomy | (a) Turner’s syndrome |
| (2) X-monosomy | (b) Klinefelter’s syndrome |
| (3) Holandric traits | (c) Down’s syndrome |
| (4) Feminized male | (d) Hypertrichosis |
Answer:
| Column I | Column II |
| (1) 21 trisomy | (c) Down’s syndrome |
| (2) X-monosomy | (a) Turner’s syndrome |
| (3) Holandric traits | (d) Hypertrichosis |
| (4) Feminized male | (b) Klinefelter’s syndrome |
![]()
5. Long Answer Questions
Question 1.
What is dihybrid cross? Explain with suitable example and checker board method.
Answer:
1. A cross which involves two pairs of alleles is called a dihybrid cross. A phenotypic ratio of 9 : 3 : 3 : 1 obtained in the F2 generation of a dihybrid cross is called a dihybrid ratio.
(2) Thus for example, when we cross a true breeding pea plant bearing round and yellow seeds with a true breeding pea plant bearing wrinkled and green seeds we get pea plants bearing round and yellow seeds in the F1 generation.
(3) When F1 plants are selfed, we get a ratio of 9 : 3 : 3 : 1 in the F2 generation, where 9 plants bear yellow round seeds, 3 plants bear yellow wrinkled seeds, 3 plants bear green round seeds and 1 plant bears green wrinkled seeds.
(4) Parents (P1) : RRYY × rryy
Gametes of P1 RY and ry
F1 generation : RrYy(Yellow round)
On selfing F1 : RrYy × RrYy
Gametes of F1 : RY, Ry, rY, ry
P2 generation:
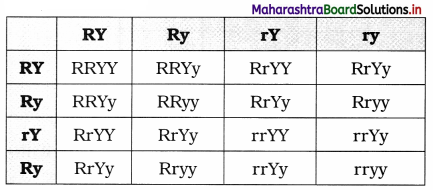
Round Yellow : 9 Round green : 3 Wrinkled yellow : 3 Wrinkled green : 1
Phenotypic ratio : 9 : 3 : 3 : 1
Genotypic ratio : 1 : 2 : 1 : 2 : 4 : 2 : 1 : 2 : 1
Question 2.
Explain with suitable example an independent assortment.
Answer:
(1) The law of independent assortment states that when hybrid possessing two or more pairs of contrasting characters bearing alleles form gametes, the alleles in each pair segregate independently of the other pair. Therefore, the inheritance of one pair of characters is independent of that of the other pair of characters.
(2) For example, when we cross a pea plant which is tall and having purple flowers with dwarf plant having white flowers we obtain all tall plants with purple flowers in F1 generation. When F1 generation are selfed, 9 : 3 : 3 : 1 ratio was obtained in F2 generation with 9 tall and purple flower, 3 tall with white flowers, 3 dwarf with purple flowers and 1 which was dwarf and white. Tallness and purple colour are dominant traits while dwarfness and white colour are recessive traits.
(i) Homozygous tall purple – TTPP
(ii) Homozygous dwarf white – ttpp
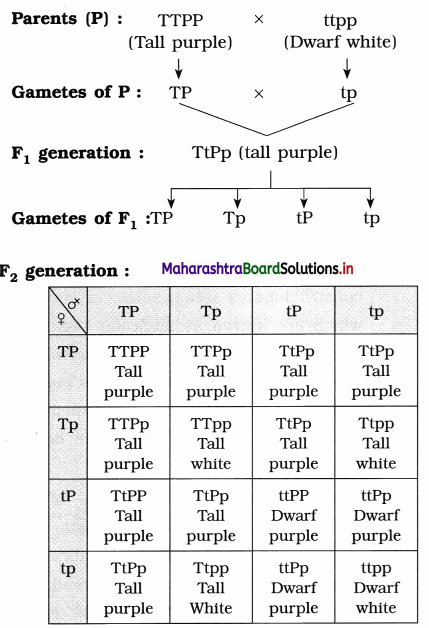
Tall purple = 9. Tall white = 3
Dwarf purple = 3, Dwarf white = 1,
Phenotypic ratio = 9 : 3 : 3 : 1
Results : The offspring of F1 generation will be in the proportion of 9 : 3 : 3 : 1, where 9 are tall purple, 3 are tall white, 3 are dwarf purple and 1 is dwarf white.
Question 3.
Define test cross and explain its significance.
Answer:
1. Definition of test cross : A cross between F1 offspring and its homozygous recessive parent is called a test cross.
2. Significance of test cross:
- Test cross can be used to find out the genotype of any plant which shows dominant characters.
- Whether the plant is homozygous or heterozygous can be understood by performing test cross.
- Test cross is used to introduce useful recessive traits in the hybrids of self- pollinated plants.
- Test cross is quicker method to improve the variety of crop plants and thus it is useful for breeders and geneticists.
- Test cross can be used for verifying the laws of inheritance.
![]()
Question 4.
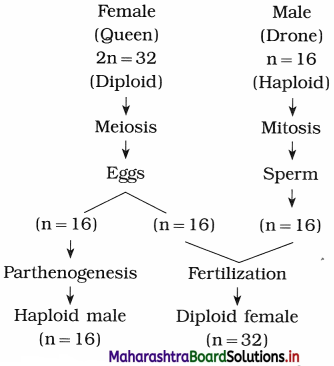
What is parthenogenesis? Explain the haplodiploid method of sex determination in honey bee.
Answer:
I. Parthenogenesis is a natural form of asexual reproduction in which growth and development of embryos occur without fertilization by sperm. In some insects like honey bees, parthenogenesis means development of an embryo from an unfertilized egg cell.
II. In honey bee:
- Sex determination is by haplodiploid system.
- Sex is determined by the number of sets of chromosomes received by an individual.
- The egg which is fertilized by sperm, becomes diploid and develops into female.
- The egg which is not fertilized develops by parthenogenesis and develops into a male.
- The queen and worker bee therefore contain 32 chromosomes. The drone, i.e. male bears 16 chromosomes.
- The sperms are produced by mitosis while eggs are produced by meiosis.
Question 5.
In the answer for inheritance of X-linked. genes, Madhav had shown carrier male. His answer was marked incorrect. Madhav was wondering why his marks were cut. Explain the reason.
Answer:
Males can never be carriers. They have single X and other Y chromosome. In X linked inheritance, the genes are present on the non-homologous region of X chromosome. Males do not have other X and hence if the genes are present on his X chromosome, they will not be suppressed in them. The Y chromosome does not have dominant gene to hide this expression as there is no homolorous region too. But in case of females, there are double X chromosomes and hence if X-linked gene is recessive, the other X can hide the expression of such X-linked gene.
Thus she becomes a carrier without showing any physical characters. She is physically normal and does not suffer from such X-linked recessive disorder. Thus, Madhav will get his answer wrong due to incorrect concept.
Question 6.
With the help of neat labelled diagram, describe the structure of chromosome.
Answer:
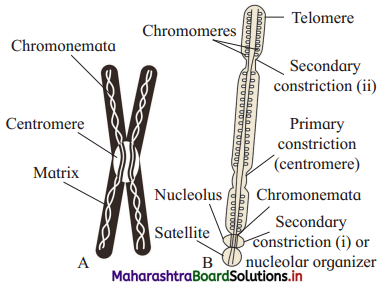
(1) A chromosome is best visible during metaphase, when it is highly condensed.
(2) Chromosome shows two identical halves, called sister chromatids. Chromatids are held together at centromere which is also called primary constriction.
(3) Primary constriction has disc shaped plate called kinetochore. This plate is useful for attachment of spindle fibres at the time of cell division.
(4) Additional narrow areas called secondary constrictions are seen in some chromosomes which are known as nucleolar organizers. They help in the formation of nucleolus. At secondary constriction (i) there is nucleolar organising region. Secondary constriction (ii) shows attachment of satellite body or SAT body.
(5) Each chromatid is made up of sub¬chromatids called chromonemata. Each chromonema consists of a long, unbranched, slender, highly coiled DNA thread. This double stranded DNA molecule extends throughout the length of the chromosome.
(6) The ends of the chromatid arms are called telomeres.
Question 7.
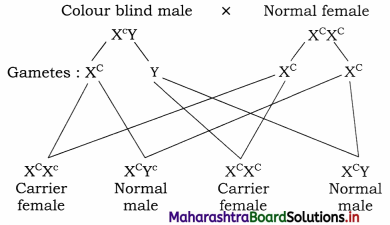
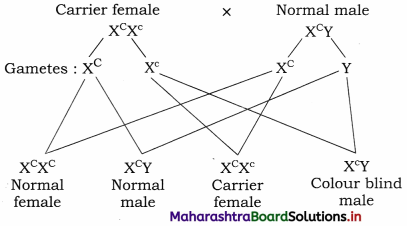
What is criss-cross inheritance? Explain with suitable example.
Answer:
Criss-cross inheritance is the type of inheritance in which the genes are passed on from father to daughter and then to her son, i.e. from male to female and from female to male (grandson). In other words, it is also said that the transmission is from the grandfather to his grandson through his daughter.
I. Inheritance of Colour blindness show criss-cross pattern.
(1) Colour blindness is a sex-linked disorder in which the person concerned cannot distinguish between red and green colours.
(2) It is recessively X-linked disorder, which is expressed in males. It is rarely seen in females.
(3) The genes for normal vision are dominant whereas those for colour blindness are recessive.
(4)
- Gene for normal vision : XC
- Gene for colour blindness : Xc
- Normal female : XCXC
- Normal male : XCY
- Colour blind female : XcXc
- Carrier female : XCXc
- Colour blind male : Xc Y
II. Crosses showing the inheritance of colour blindness:
(i) A cross between normal female and colour-blind male.
(ii) A cross of carrier female with normal male.
(1) Normal female with Colour blind male. Such cross produces 50% carrier daughters and 50% normal sons.
(2) Carrier female with normal male. Such a cross produces 25% normal daughters, 25% normal sons, 25% carrier daughters and 25% colour blind sons.
(3) Colour blind father transmits the disorder to his grandson through his carrier daughter. The inheritance of characters from the father to his grandson through his daughter is called criss-cross inheritance.
![]()
Question 8.
Describe the different types of chromosomes.
Answer:
I. Chromosomes are classified into the following four types according to the position of the centromere in them:
(1) Metacentric : In metacentric chromosome, the centromere is situated in the middle of the chromosome. The two arms of the chromosome are nearly equal. It appears ‘V’-shaped during anaphase.
(2) Sub-metacentric : In sub-metacentric chromosome, the centromere is situated some distance away from the middle. Due to this, one arm of the chromosome is shorter than the other. It appears T-shaped during anaphase.
(3) Acrocentric : In acrocentric chromosome, the centromere is situated near the end of the chromosome. One arm of the acrocentric chromosome is very short while the other is long making it appear like ‘J’-shaped during anaphase.
(4) Telocentric : In telocentric chromosome, the centromere is situated at the tip of the chromosome. Telocentric chromosome has only one arm thus it appears rod-shaped.
II. Based on the functions, chromosomes are divided into autosomes and allosomes. Autosomes are somatic chromosomes which decide the body characters. Allosomes are sex chromosomes which decide the sex of the individual.
Maharashtra State Board 12th Std Biology Textbook Solutions
- Reproduction in Lower and Higher Plants Class 12 Biology Textbook Solutions
- Reproduction in Lower and Higher Animals Class 12 Biology Textbook Solutions
- Inheritance and Variation Class 12 Biology Textbook Solutions
- Molecular Basis of Inheritance Class 12 Biology Textbook Solutions
- Origin and Evolution of Life Class 12 Biology Textbook Solutions
- Plant Water Relation Class 12 Biology Textbook Solutions
- Plant Growth and Mineral Nutrition Class 12 Biology Textbook Solutions
- Respiration and Circulation Class 12 Biology Textbook Solutions